Abstract
Serine hydroxymethyl transferase (SHMT) is a pyridoxal phosphate (PLP)-dependent enzyme that catalyzes the reversible conversion of serine and tetrahydrofolate to glycine and methylenetetrahydrofolate. We have identified a single gene encoding SHMT in the genome of Trichomonas vaginalis, an amitochondriate, deep-branching unicellular protist. The protein possesses a putative N-terminal hydrogenosomal presequence and was shown to localize to hydrogensomes by immunofluorescence analysis, providing evidence of amino acid metabolism in this unusual organelle. In contrast to the tetrameric SHMT that exists in the mammalian host, we found that the T. vaginalis SHMT is a homodimer, as found in prokaryotes. All examined SHMT contain an 8-amino-acid conserved sequence, VTTTTHKT, containing the active-site lysyl residue (Lys 251 in TvSHMT) that forms an internal aldimine with PLP. We mutated this Lys residue to Arg and Gln and examined structural and catalytic properties of the wild-type and mutant enzymes in comparison to that reported for the mammalian protein. The oligomeric structure of the mutant K251R and K251Q TvSHMT was not affected, in contrast to that observed for comparable mutations in the mammalian enzyme. Likewise, contrary to that observed for mammalian SHMT, the catalytic activity of K251R TvSHMT was unaffected in the presence of PLP. The K251Q TvSHMT, however, was found to be inactive. These studies indicate that the active site of the parasite enzyme is distinct from its prokaryotic and eukaryotic counterparts and identify TvSHMT as a potential drug target.
Serine hydroxymethyl transferase (SHMT) reversibly converts serine and tetrahydrofolate (THF) to glycine and 5-10-methylene tetrahydrofolate, the principal pathway for incorporation of one-carbon units in the biosynthesis of numerous cell metabolites (27). Most SHMTs studied require pyridoxal-5′-phosphate (PLP) and belong to the α-family (4) of PLP enzymes. Found in high levels in rapidly proliferating cells compared with resting cells, this enzyme has been proposed to be a potential target for the development of anticancer and antimicrobial agents (1).
SHMT activity is present in prokaryotes and eukaryotes, with eukaryotic cells containing both cytosolic and mitochondrial forms (5) encoded by separate genes (18). A chloroplast isoform has also been reported to be present in plants (7). Nearly all prokaryotic SHMTs exist as homodimers (3, 8, 25), and eukaryotic SHMTs exist as homotetramers (22, 32). SHMT activity has been detected in trypanosomatids and apicomplexans (2, 11, 12). The enzyme has been crystallized from several sources (8, 23, 24, 25), providing a basis for correlating function and structure.
To date, no information on this enzyme in Trichomonas vaginalis, a deep-branching, microaerophilic, unicellular eukaryote is available. T. vaginalis is a parasitic protist that adheres to the epithelial lining of the urogenital tract of humans (6). It is the causative agent of trichomoniasis, one of the most common nonviral sexually transmitted diseases worldwide. These parasites contain a unique organelle, the hydrogenosome, and lack classic mitochondria (21). In contrast to mitochondria, hydrogenosomes do not contain cytochromes and perform neither the tricarboxylic acid cycle nor electron-transport-linked oxidative phosphorylation. Hydrogenosomes are involved in iron-sulfur cluster assembly (29, 30) and convert pyruvate or malate under anoxic conditions to hydrogen, acetate, and carbon dioxide, with the concomitant production of ATP by substrate level phosphorylation (15).
This study reports the identification and characterization of T. vaginalis SHMT. The enzyme was shown to possess a putative hydrogenosomal presequence and to be targeted to the hydrogenosome. Structural and catalytic properties of the enzyme have been examined and compared to those of prokaryotic and eukaryotic counterparts.
MATERIALS AND METHODS
Parasite cell culture.
Trichomonas vaginalis strains T1 and G3 were grown in Diamond's medium supplemented with 10% (vol/vol) horse serum and iron as described previously (22).
Plasmid construction.
We designed primers SHMTNF and SHMTXR (see the table in the supplemental material) to amplify the complete open reading frame (ORF) of the gene encoding the SHMT protein from the T. vaginalis genomic DNA for in-frame cloning into the pET-29B expression vector (Novagen) using NdeI and XhoI restriction sites. The resulting recombinant C-terminally His-tagged fusion protein was expressed in Escherichia coli BL21(DE3) cells (Invitrogen). For expressing the protein in T. vaginalis, we PCR amplified the gene with primer pairs SHMTNF/SHMTTvKR (see the table in the supplemental material). The PCR fragment was then cloned into master-neo-(HA)2 plasmid (15) to generate the construct to transfect T. vaginalis. Point mutations were introduced (K251R and K251Q) at Lys-251 (Fig. 1) by site-directed mutagenesis using the primer sets K251RFP/K251RRP and K251QFP/K251QRP (see the table in the supplemental material), respectively. These mutant proteins were expressed in T. vaginalis and in bacteria to purify the recombinant proteins.
FIG. 1.
Alignment of SHMT amino acid sequences. The alignment of amino acid sequences deduced from the gene encoding SHMT from T. vaginalis (Tv; TVAG_109540), Homo sapiens (mHs; P34897), and E. coli (Ec; POA825) using the software CLUSTALX is shown. Asterisks and dots represent identities and similarities, respectively. The solid black line indicates an 8-amino-acid conserved sequence containing the active-site lysine residue (boxed). The predicted hydrogenosomal presequence is marked in boldface type in the T. vaginalis protein sequence.
Selectable transfection of T. vaginalis.
Electroporation of T. vaginalis strain T1 was carried out as described previously (14) with 50 μg of circular plasmid DNA. Transfectants were selected with 100 μg/ml of G418 (Sigma) prior to crude fractionation and organelle purification.
Immunofluorescence microscopy.
Live T. vaginalis cells were washed with warm Diamond's media and allowed to attach to coverslips for 30 min in a humidifying chamber. The cells were then washed with warm 1× phosphate-buffered saline and fixed with 3.5% formalin for 20 min at room temperature. The C-terminally hemagglutinin (HA)-tagged SHMT protein and Hsp70 were visualized using mouse anti-HA monoclonal antibody (Sigma) and rabbit anti-Hsp70 polyclonal antibody as primary antibodies and secondary Alexa Fluor-488 donkey anti-mouse (green) and Alexa Fluor-594 donkey anti-rabbit (red) antibodies (Invitrogen). Cells were visualized at ×100 magnification using an Axioscop2 (Zeiss). Images were processed with Axiovision v. 3.2 software (Zeiss).
Isolation of hydrogenosomes.
Hydrogenosomes were prepared as previously described by Bradley et al. (9). The organelles were then resuspended in a buffer containing 1% Triton X-100, 20 mM Tris-HCl, pH 7.5, 2% sucrose, 250 mM NaCl, 5 mg/ml leupeptin, and 25 mg/ml Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) and incubated on ice for 30 min, with occasional vortexing every 5 min. The clear hydrogenosomal lysate was finally obtained after centrifugation at 16,000 × g for 30 min at 4°C.
Sucrose density gradient analysis.
Hydrogenosomal extracts (0.5 mg) from transfected T. vaginalis cells (1.0 ml) were loaded onto linear sucrose gradients (2 to 20%) in 20 mM Tris-HCl at pH 7.5 containing 1% Triton X-100, 250 mM NaCl, TLCK, and leupeptin prepared in 25- by 89-mm centrifugation tubes (Beckman) made with a Gradient Master (BioComp Instruments) and were immediately subjected to centrifugation at 38,000 rpm for 16 h at 4°C with an SW41 rotor (Beckman). Ten serial fractions were collected from the top of the gradients using a Piston Gradient Fractionator (BioComp Instruments), tricarboxylic acid precipitated, and subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by immunoblot analysis using anti-HA antibodies. Marker proteins (Amersham Bioscience) bovine serum albumin (67 kDa), lactate dehydrogenase (140 kDa), catalase (232 kDa), and ferritin (440 kDa) were subjected to sucrose gradients under conditions similar to those described above for the marker proteins and detected by Coomassie staining after SDS-PAGE.
Expression of recombinant proteins.
The pET-SHMTwt, pET-K251R, and pET-K251Q plasmids were transformed into E. coli BL21(DE3) cells and selected with 30 μg/ml kanamycin in LB media. For the expression of the wild-type and mutant proteins, 500-ml cultures were grown at 37°C to an A600 of 0.8 before induction with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside for 3 h at 37°C. Harvested cells were then resuspended in a buffer containing 1 mg/ml of lysozyme, 0.05 M NaH2PO4, 0.3 M NaCl, 50 μM PLP, 10 mM imidazole, protease inhibitor cocktail (Roche Diagnostics), 5 mg/ml leupeptin, and 25 mg/ml TLCK for 20 min at 4°C and sonicated. The insoluble material was pelleted at 10,000 × g for 30 min at 4°C, and the resulting lysate was applied to a nickel-nitrilotriacetic acid-agarose column. The column was washed four times with a buffer containing 0.05 M NaH2PO4, 0.3 M NaCl, and 20 mM imidazole, pH 8.0. Specifically bound proteins were eluted with the above buffer containing 0.25 M imidazole. The fractions containing SHMT were pooled, and glycerol and dithiothreitol (DTT) were added to final concentrations of 8% and 1 mM, respectively. Excess imidazole was removed by dialysis against 25 mM potassium phosphate buffer, pH 7.6, containing 50 mM NaCl, 1 mM EDTA, 8% glycerol, and 1 mM DTT. The purity of the purified proteins was checked by SDS-PAGE.
Enzyme activity.
SHMT activity of the purified recombinant proteins (wild type and mutants) were determined as reported earlier (2). The enzyme activity was tested directly by monitoring conversion of radioactive carbon from serine to methylenetetrahydrofolate. Briefly, the assay mixture consisted of 50 mM Tris-HCl, pH 8.0, 1 mM DTT, 0.25 mM PLP, 2.5 mM EDTA, 0.2 mM l-Ser, 0.6 nmol [14C]Ser, and varied concentrations of THF. The reaction was started by adding ∼2 mg of the enzyme. After incubation at 37°C for 20 min, the reaction mixture was streaked onto 2-cm2 Whatman DE-81 paper. Unreacted serine was desorbed by washing the filter for 30 min (with three changes) in distilled water. The radioactivity associated with methylenetetrahydrofolate was determined by drying the washed filter and subjecting it to liquid scintillation spectroscopy.
Homology modeling.
A structural model of the T. vaginalis SHMT (TvSHMT) enzyme (TVAG_109540) was created using the SWISS-MODEL comparative protein modeling server (28). The coordinates from the rabbit SHMT (PDB identification no. 1LS3) (16) were used as a structural template. The N-glycine-[3-hydroxy-2-methyl-5-phosphonooxymethyl-pyridin-4-yl-methane] (PLG) substrate was modeled into the SHMT by structurally aligning the T. vaginalis SHMT model with the structure of E. coli SHMT (PDB identification no. 1DFO) (25) using lsqman (20) from the Uppsala Software Factory. Pymol (http://www.pymol.org) was used to study models and generate figures (PyMOL Molecular Graphics System; DeLano Scientific, San Carlos, CA) (13).
RESULTS
Identification of a putative gene encoding SHMT.
To identify the T. vaginalis SHMT, we searched the ∼7× coverage of the Trichomonas vaginalis genome database (http://tigrblast.tigr.org/er-blast/index.cgi?project=tvg) with both prokaryotic and eukaryotic SHMT sequences, and only one gene (locus TVAG_109540 [83992.m00192]) was found. Comparison of deduced amino acid sequences of this TvSHMT revealed 49% identity and 66% similarity with mitochondrial SHMT from Homo sapiens (accession number P34897) and 46% identity and 62% similarity with its counterpart in E. coli (accession number POA825) (Fig. 1) using CLUSTAL X. The predicted ORF of the TvSHMT protein consisted of 451 amino acids with a calculated molecular mass of ∼50 kDa. Expression of the TvSHMT gene was confirmed by reverse transcription-PCR (data not shown).
SHMT localizes in the hydrogenosomes of T. vaginalis.
To investigate the subcellular localization of the SHMT protein, C-terminally HA-tagged proteins were expressed in T. vaginalis and localized using immunofluorescence analyses. The protein was found to colocalize with Hsp70, a marker for the hydrogenosomes, demonstrating that the protein is found in this unusual organelle (Fig. 2A). Western analyses of hydrogenosomes isolated from T. vaginalis cells expressing the tagged protein with anti-HA antibody also indicated that SHMT is localized to the hydrogenosomes (data not shown). Moreover, SHMT activity was detected in hydrogenosomal extracts prepared from untransfected cells, further confirming this result (Fig. 2B).
FIG. 2.
Cellular localization of SHMT in T. vaginalis transfectants. (A) Cells expressing HA-tagged SHMT were stained for immunofluorescence microscopy using a mouse HA-tagged antibody. T. vaginalis anti-Hsp70 was used as a positive control for hydrogenosomal localization (10). The nucleus (blue) was stained with 4′,6′-diamidino-2-phenylindole (DAPI), SHMT was stained with mouse anti-HA (green), and Hsp70 was stained with rabbit anti-Hsp70 (red). Merged images demonstrate the colocalization of SHMT and Hsp70. PC indicates the phase-contrast image. (B) SHMT activity using variable amounts of hydrogenosomal and cytosolic extracts prepared using untransfected T. vaginalis cells in the presence of 2 mM THF and 0.25 mM externally added PLP. Enzyme activity was expressed as pmol of methylenetetrahydrofolate formed per min per mg of total protein. Solid bars (A) represent activity in hydrogenosomal extracts; open bars (B) represent activity in cytosolic extracts. Error bars reflect standard deviations of results from three measurements.
Characterization of TvSHMT.
We have characterized TvSHMT to determine whether it might be a good candidate for a drug target to treat trichomoniasis. Most of the amino acids known to be associated with the catalytic site of other SHMT, such as Lys-251 (31), which is predicted to interact with PLP, are conserved in TvSHMT (Fig. 1). We mutated the Lys-251 residue to Arg or Gln to ascertain whether differences exist in structural and catalytic properties of TvSHMT relative to its human counterpart.
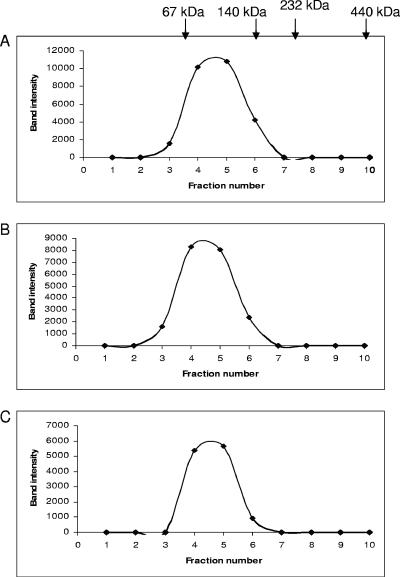
Wild-type TvSHMT (SHMTwt) and mutant (K251R and K251Q) enzymes were expressed in T. vaginalis with a C-terminal HA tag. Hydrogenosomes isolated from the three T. vaginalis transfectants were solubilized in nonionic, nondenaturing detergent, Triton X-100, and the cleared lysates were subjected to 2 to 20% sucrose gradient ultracentrifugation. The sucrose gradient fractions were then analyzed by SDS-PAGE, followed by immunoblotting using anti-HA antibody. SHMTwt, K251R, and K251Q were all found to fractionate in 8 to 10% sucrose, between 67-kDa and 140-kDa markers, indicating that TvSHMT exists as a dimer in the hydrogenosomes and that mutating the Lys residue to Arg or Gln does not alter its oligomeric structure (Fig. 3). Analytical ultracentrifugation results also indicate that recombinant SHMTwt, K251R, and K251Q proteins form dimers (data not shown).
FIG. 3.
Sucrose density gradient analysis of hydrogenosomal lysates. Hydrogenosomal lysates from cells transfected with plasmids expressing C-terminally HA-tagged SHMTwt (A), K251R (B), and K251Q (C) proteins were fractionated on linear sucrose gradients (2 to 20%), tricarboxylic acid precipitated, subjected to 10% SDS-PAGE, and analyzed by immunoblotting using anti-HA antibody. Band intensity was plotted against fraction number. Arrows indicate the positions of molecular mass standards (bovine serum albumin, 67 kDa; lactate dehydrogenase, 140 kDa; catalase, 232 kDa; ferritin, 440 kDa).
Catalytic activity of the wild-type and mutant recombinant proteins.
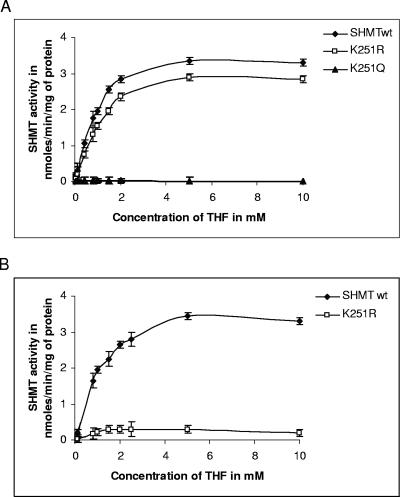
To determine whether TvSHMT is active and to compare the activity of SHMTwt, K251R, and K251Q, these recombinant proteins were expressed in BL21(DE3) cells as C-terminally His-tagged proteins. The proteins were purified using Ni-nitrilotriacetic acid column chromatography and assessed to be >98% pure by Coomassie blue-stained SDS-PAGE (data not shown). We then determined the catalytic activity of the recombinant SHMTwt, K251R, and K251Q proteins in the presence and absence of PLP, using a fixed concentration of l-Ser (0.2 mM) and variable concentrations of THF as substrates. In the presence of PLP (0.25 mM), the SHMT activity of K251R (apparent Vmax, 3.4 nmol/mg/min) was comparable to that of the wild-type enzyme (apparent Vmax, 3.8 nmol/mg/min) (Fig. 4A). However, in the absence of PLP, the mutant enzyme K251R (apparent Vmax, 0.3 nmol/mg/min) was found to have a very low activity, with a kcat value about 250-fold lower than that of the wild-type enzyme (Fig. 4B). Furthermore, the mutant enzyme K251Q was found to be inactive in both the presence (Fig. 4A) and absence of PLP (data not shown). The kinetic parameters of the wild-type and K251R enzymes are summarized in Table 1.
FIG. 4.
Substrate kinetics of the reaction catalyzed by SHMT. (A) Reaction kinetics assessed using 1 μM purified SHMTwt, K251R, and K251Q proteins in the presence of 0.25 mM externally added PLP using variable concentration of THF. (B) Substrate kinetics of the reaction using SHMTwt and K251R under the same conditions in the absence of externally added PLP. The enzyme activity was measured by determining the incorporation of the radioactive label into methylenetetrahydrofolate by liquid scintillation spectroscopy. Error bars represent standard deviations of results from three measurements.
TABLE 1.
Kinetic properties of SHMTwt and K251Ra
| Enzyme | Result with:
|
|||||
|---|---|---|---|---|---|---|
| 0.25 mM PLP
|
No PLP
|
|||||
| Apparent Km (mM) | kcat (s−1) | kcat/Km (s−1 M−1) | Apparent Km (mM) | kcat (s−1) | kcat/Km (s−1 M−1) | |
| SHMTwt | 0.89 | 1.3 | 1.46 × 103 | 1.0 | 1.29 | 1.29 × 103 |
| K251R | 1.16 | 1.13 | 0.97 × 103 | 0.3 | 0.005 | 0.02 × 103 |
The enzymatic assay was performed as described in Materials and Methods. Kinetic parameters were calculated using varying concentrations of THF in the presence of 0.25 mM PLP or in the absence of externally added PLP.
Homology model of TvSHMT protein.
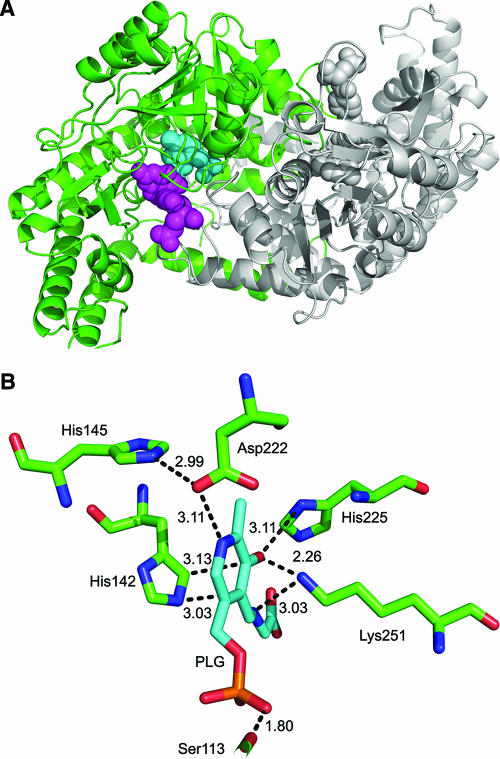
To gain insight regarding the potential of exploiting TvSHMT as a drug target, we modeled residues 14 to 451 of the parasite enzyme as a dimer based on the coordinates of the crystal structure of rabbit SHMT protein (16) (Fig. 5A). All mammalian SHMTs are highly conserved, and the predicted three-dimensional structure of human SHMT can be overlaid on the sheep SHMT (scSHMT), making it likely that comparisons with scSHMT are relevant to the host enzyme. Each monomer in our model is composed of an N-terminal domain, a large central domain, and a C-terminal domain. The short N-terminal domain (residues 14 to 49) is primarily α-helical. The central or the “large” domain (residues 50 to 300) binds PLG, an analogue of PLP and FFO (5-formyl tetrahydrofolate), an analogue of THF, has most of the active-site residues, and folds into an α-β-α sandwich containing 9 α-helices wrapped around a 7-stranded mixed β-sheet. Finally, the C-terminal domain (residues 301 to 451) folds into an α-β complex. The proposed amino acid residues interacting directly with PLP of TvSHMT are Ser-113, His-142, Asp-222, His-225, and Lys-251. His-145 is found to be hydrogen bonded to Asp-222, which in turn affects the interaction of Asp-222 with PLP (Fig. 5B).
FIG. 5.
Proposed homology model for TvSHMT based on the 1LS3 structure. (A) The proposed dimer is comprised of two monomers (marked in green and gray). PLG (an analogue of PLP) and 5-formyl tetrahydrofolate (an analogue of THF) are shown in a space-fill representation and are colored in cyan and magenta, respectively. (B) A view of the active site of TvSHMT showing interactions of Ser-113, His-142, His-145, Asp-222, His-225, and Lys-251, with PLG marked in cyan.
DISCUSSION
We have identified and characterized a homolog of SHMT from the eukaryotic pathogen T. vaginalis. This protein is encoded by a single gene with an ORF of 1,353 bp and a predicted protein of 451 amino acid residues. The protein is found to be localized to the hydrogenosome (Fig. 2A and B). Our earlier studies (20a) indicate the presence of components of glycine decarboxylase complex in the hydrogenosomes providing the first evidence of amino acid metabolism in this organelle. Both glycine decarboxylase complex and SHMT are vital for the interconversion of glycine and serine (27). Thus, the presence of hydrogenosomal SHMT further supports this new predicted function of this unusual organelle in trichomonads.
SHMT is a highly conserved protein and exists in different isoforms encoded by different genes. In most eukaryotes, both cytosolic and mitochondrial isoforms are found. However, only a cytosolic isoform is found in the parasite Trypanosoma cruzi (12). Likewise, only a single gene encoding the cytosolic isoform of the malarial SHMT has been identified (2). On the other hand, the trypanosome Crithidia fasciculata has three isoforms found in the mitochondrion, cytosol, and glycosome (11). Cytosolic (SHMT-S) and mitochondrial (SHMT-L) isoforms are also found in the kinetoplastid parasite Leishmania (17). We found only one SHMT gene in T. vaginalis that encodes a hydrogenosomal protein. The apparent lack of a cytosolic form of SHMT implies that the interconversion of glycine to serine occurs exclusively in hydrogenosomes in the parasite. However, we cannot exclude the possibility that another gene, encoding a cytosolic TvSHMT, is simply missing from the 7× coverage of the T. vaginalis genome sequence. If so, the gene is likely to be highly divergent, as only one gene copy is detected in the genome by hybridization analyses (data not shown).
SHMT has been widely studied in many living systems (e.g., bacteria, humans, sheep, kinetoplastids, and plants) with regard to its expression, subunit composition, and structural folding patterns. Mitochondrial SHMTs studied to date are usually found to exist as tetramers. Sucrose density gradient analysis of the hydrogenosomal TvSHMT indicates that the protein exists as a dimer, as is found in E. coli and Bacillus stearothermophilus (8), which differentiates it from its human counterparts that exist as tetramers.
Structural features that are fundamental to the maintenance of the tetrameric structure of all the mammalian SHMTs include the His-158 residue (Fig. 1, human SHMT) which hydrogen bonds to another His-158 residue to create the second dimeric interface, facilitating the assembly of the symmetric tetrameric quaternary structure. TvSHMT and E. coli SHMT (EcSHMT) lack this histidine residue and instead have a glycine that would not facilitate hydrogen bonding between the dimers to form tetramers (23). Other important structural features to the tetramer-stabilizing interactions are two insertions present in the mammalian enzymes. The first insertion is KRISATSI beginning at residue Lys-181. This insertion is changed to KKVSSSSI in TvSHMT. The second insertion is RKGVKAVDPKTGREIPY, starting at residue Arg-293. This insertion is incomplete in both TvSHMT and EcSHMT (Fig. 1). Scarsdale et al. (25) proposed that EcSHMT could not form the same tetrameric quaternary structure observed for mammalian SHMT because, besides not having a residue equivalent to His-158, it lacks the tetramer-stabilizing interactions. We observed that TvSHMT possesses the first tetramer stabilizing insertion; however, 4 of the 8 amino acid residues are different. The parasite enzyme was also found to lack the second tetramer-stabilizing insertion along with the central histidine residue. These data are consistent with the proposed role of these insertions in tetramer stabilization. Moreover, our experimental evidence together with primary sequence analyses demonstrate that the oligomeric structure of the T. vaginalis protein is distinctly different from its counterpart in the human host.
All characterized SHMT contain an 8-amino-acid conserved sequence, VTTTTHKT, containing the active-site lysyl residue (K) that forms the internal aldimine with PLP (Fig. 1) (e.g., Lys-251 in TvSHMT and Lys-229 in EcSHMT). Mutation of this Lys to Arg or Gln in TvSHMT did not alter the oligomeric structure of the proteins (Fig. 3), nonetheless a complete loss of activity was observed in the K251Q mutant. The K251R mutant retained activity comparable to that of the wild-type enzyme in the presence of 0.25 mM PLP (Fig. 4A) but showed a very low activity in the absence of PLP (Fig. 4B; Table 1). These data are consistent with the oligomeric structure of the protein not requiring K251 for stabilization and indicate that the effect on activity when this residue is mutated can be overcome by interaction of other residues with PLP (Fig. 5B). In contrast, mutation of Lys-229 to His or Arg in dimeric E. coli recombinant SHMT resulted in the loss of catalytic activity and the inability to bind PLP, except in its free aldehyde form (26). When the EcSHMT Lys-229 was mutated to Gln (K229Q), binding to external aldimine and catalysis of a single turnover was observed. Finally, with regard to differences between the mammalian and T. vaginalis enzymes, mutation of the corresponding Lys to Gln or Arg in scSHMT converted the tetrameric enzyme to an inactive dimer with no bound PLP (19). Taken together, these data reveal structural differences between the active sites of mammalian and trichomonad SHMT that might be exploited in drug design to specifically inhibit the parasite enzyme.
Although detailed structural analysis of the parasite protein awaits the resolution of its crystal structure, the identity of the T. vaginalis SHMT and initial characterization of its domain structure and active site are first steps toward deciphering differences between the parasite protein and its human counterpart. Moreover, further analyses of the catalytically active form of TvSHMT will facilitate the search for specific inhibitors that may allow the enzyme to be exploited as a potential drug target. Detailed analysis of the structural changes induced in K251Q, resulting in a catalytically inactive enzyme, should also be useful for screening drugs which stabilize its structure, thus reducing the activity of the enzyme.
Supplementary Material
Acknowledgments
We thank our colleagues in the lab for helpful discussions.
This work was supported by National Institutes of Health (NIH) grants to P.J.J., a Burroughs-Wellcome Molecular Parasitology Award to P.J.J., and an NIH Microbial Pathogenesis Training Grant (2-T32-AI-07323) stipend to M.T.B., and a UCLA-IGERT Bioinformatics Training (NSF DGE-9987641) stipend to S.A.S.
Footnotes
Published ahead of print on 15 September 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Agrawal, S., A. Kumar, V. Srivastava, and B. N. Mishra. 2003. Cloning, expression, activity and folding studies of serine hydroxymethyltransferase: a target enzyme for cancer chemotherapy. J. Mol. Microbiol. Biotechnol. 6:67-75. [DOI] [PubMed] [Google Scholar]
- 2.Alfadhli, S., and P. K. Rathod. 2000. Gene organization of a Plasmodium falciparum serine hydroxymethyltransferase and its functional expression in Escherichia coli. Mol. Biochem. Parasitol. 110:283-291. [DOI] [PubMed] [Google Scholar]
- 3.Angelaccio, S., R. Chiaraluce, V. Consalvi, B. Buchenau, L. Giangiacomo, F. Bossa, and R. Contestabile. 2003. Catalytic and thermodynamic properties of tetrahydromethanopterin-dependent serine hydroxymethyltransferase from Methanococcus jannaschii. J. Biol. Chem. 278:41789-41797. [DOI] [PubMed] [Google Scholar]
- 4.Appaji Rao, N., M. Ambili, V. R. Jala, H. S. Subramanya, and H. S. Savithri. 2003. Structure-function relationship in serine hydroxymethyltransferase. Biochim. Biophys. Acta 1647:24-29. [DOI] [PubMed] [Google Scholar]
- 5.Appling, D. R. 1991. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 5:2645-2651. [DOI] [PubMed] [Google Scholar]
- 6.Bastida-Corcuera, F. D., C. Y. Okumura, A. Colocoussi, and P. J. Johnson. 2005. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 4:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauwe, H., and U. Kolukisaoglu. 2003. Genetic manipulation of glycine decarboxylation. J. Exp. Bot. 54:1523-1535. [DOI] [PubMed] [Google Scholar]
- 8.Bhavani, S., V. Trivedi, V. R. Jala, H. S. Subramanya, P. Kaul, V. Prakash, N. Appaji Rao, and H. S. Savithri. 2005. Role of Lys-226 in the catalytic mechanism of Bacillus stearothermophilus serine hydroxymethyltransferase-crystal structure and kinetic studies. Biochemistry 44:6929-6937. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, P. J., C. J. Lahti, E. Plumper, and P. J. Johnson. 1997. Targeting and translocation of proteins into the hydrogenosome of the protist Trichomonas: similarities with mitochondrial protein import. EMBO J. 16:3484-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bui, E. T., P. J. Bradley, and P. J. Johnson. 1996. A common evolutionary origin for mitochondria and hydrogenosomes. Proc. Natl. Acad. Sci. USA 93:9651-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capelluto, D. G., U. Hellman, J. J. Cazzulo, and J. J. Cannata. 1999. Purification and partial characterization of three isoforms of serine hydroxymethyltransferase from Crithidia fasciculata. Mol. Biochem. Parasitol. 98:187-201. [DOI] [PubMed] [Google Scholar]
- 12.Capelluto, D. G., U. Hellman, J. J. Cazzulo, and J. J. Cannata. 2000. Purification and some properties of serine hydroxymethyltransferase from Trypanosoma cruzi. Eur. J. Biochem. 267:712-719. [DOI] [PubMed] [Google Scholar]
- 13.DeLano, W. L. 2002. The PyMOL user's manual. DeLano Scientific, San Carlos, Calif.
- 14.Delgadillo, M. G., D. R. Liston, K. Niazi, and P. J. Johnson. 1997. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 94:4716-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyall, S. D., C. M. Koehler, M. G. Delgadillo-Correa, P. J. Bradley, E. Plumper, D. Leuenberger, C. W. Turck, and P. J. Johnson. 2000. Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol. Cell. Biol. 20:2488-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu, T. F., J. N. Scarsdale, G. Kazanina, V. Schirch, and H. T. Wright. 2003. Location of the pteroylpolyglutamate-binding site on rabbit cytosolic serine hydroxymethyltransferase. J. Biol. Chem. 278:2645-2653. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon, D., A. Foucher, I. Girard, and M. Ouellette. 13. July 2006. Stage specific gene expression and cellular localization of two isoforms of the serine hydroxymethyltransferase in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 150:63-71. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 18.Garrow, T. A., A. A. Brenner, V. M. Whitehead, X. N. Chen, R. G. Duncan, J. R. Korenberg, and B. Shane. 1993. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal localization. J. Biol. Chem. 268:11910-11916. [PubMed] [Google Scholar]
- 19.Jala, V. R., N. Appaji Rao, and H. S. Savithri. 2003. Identification of amino acid residues, essential for maintaining the tetrameric structure of sheep liver cytosolic serine hydroxymethyltransferase, by targeted mutagenesis. Biochem. J. 369:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleywegt, G. J. 1996. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D 52:842-857. [DOI] [PubMed] [Google Scholar]
- 20a.Mukherjee, M., M. T. Brown, A. G. McArthur, and P. J. Johnson. 2006. Proteins of the glycine decarboxylase complex in the hydrogenosome of Trichomonas vaginalis. Eukaryot. Cell 5:2062-2071. [DOI] [PMC free article] [PubMed]
- 21.Muller, M. 1993. The hydrogenosome. J. Gen. Microbiol. 139:2879-2889. [DOI] [PubMed] [Google Scholar]
- 22.Quon, D. V., M. G. Delgadillo, A. Khachi, S. T. Smale, and P. J. Johnson. 1994. Similarity between a ubiquitous promoter element in an ancient eukaryote and mammalian initiator elements. Proc. Natl. Acad. Sci. USA 91:4579-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renwick, S. B., K. Snell, and U. Baumann. 1998. The crystal structure of human cytosolic serine hydroxymethyltransferase: a target for cancer chemotherapy. Structure 6:1105-1116. [DOI] [PubMed] [Google Scholar]
- 24.Scarsdale, J. N., G. Kazanina, S. Radaev, V. Schirch, and H. T. Wright. 1999. Crystal structure of rabbit cytosolic serine hydroxymethyltransferase at 2.8 A resolution: mechanistic implications. Biochemistry 38:8347-8358. [DOI] [PubMed] [Google Scholar]
- 25.Scarsdale, J. N., S. Radaev, G. Kazanina, V. Schirch, and H. T. Wright. 2000. Crystal structure at 2.4 A resolution of E. coli serine hydroxymethyltransferase in complex with glycine substrate and 5-formyl tetrahydrofolate. J. Mol. Biol. 296:155-168. [DOI] [PubMed] [Google Scholar]
- 26.Schirch, D., S. Delle Fratte, S. Iurescia, S. Angelaccio, R. Contestabile, F. Bossa, and V. Schirch. 1993. Function of the active-site lysine in Escherichia coli serine hydroxymethyltransferase. J. Biol. Chem. 268:23132-23138. [PubMed] [Google Scholar]
- 27.Schirch, V., and D. M. Szebenyi. 2005. Serine hydroxymethyltransferase revisited. Curr. Opin. Chem. Biol. 9:482-487. [DOI] [PubMed] [Google Scholar]
- 28.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutak, R, P. Dolezal, H. L. Fiumera, I. Hrdy, A. Dancis, M. Delgadillo-Correa, P. J. Johnson, M. Muller, and J. Tachezy. 2004. Mitochondrial-type assembly of FeS centers in the hydrogenosomes of the amitochondriate eukaryote Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 101:10368-10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachezy, J, L. B. Sanchez, and M. Muller. 2001. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol. Biol. Evol. 18:1919-1928. [DOI] [PubMed] [Google Scholar]
- 31.Talwar, R., J. R. Jagath, A. Datta, V. Prakash, H. S. Savithri, and N. A. Rao. 1997. The role of lysine-256 in the structure and function of sheep liver recombinant serine hydroxymethyltransferase. Acta Biochim. Pol. 44:679-688. [PubMed] [Google Scholar]
- 32.Venkatesha, B., J. B. Udgaonkar, N. A. Rao, and H. S. Savithri. 1998. Reversible unfolding of sheep liver tetrameric serine hydroxymethyltransferase. Biochim. Biophys. Acta 1384:141-152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.