Abstract
The transition from yeast-like to filamentous growth in the biotrophic fungal phytopathogen Ustilago maydis is a crucial event for pathogenesis. Previously, we showed that fatty acids induce filamentation in U. maydis and that the resulting hyphal cells resemble the infectious filaments observed in planta. To explore the potential metabolic role of lipids in the morphological transition and in pathogenic development in host tissue, we deleted the mfe2 gene encoding the multifunctional enzyme that catalyzes the second and third reactions in β-oxidation of fatty acids in peroxisomes. The growth of the strains defective in mfe2 was attenuated on long-chain fatty acids and abolished on very-long-chain fatty acids. The mfe2 gene was not generally required for the production of filaments during mating in vitro, but loss of the gene blocked extensive proliferation of fungal filaments in planta. Consistent with this observation, mfe2 mutants exhibited significantly reduced virulence in that only 27% of infected seedlings produced tumors compared to 88% tumor production upon infection by wild-type strains. Similarly, a defect in virulence was observed in developing ears upon infection of mature maize plants. Specifically, the absence of the mfe2 gene delayed the development of teliospores within mature tumor tissue. Overall, these results indicate that the ability to utilize host lipids contributes to the pathogenic development of U. maydis.
Ustilago maydis causes a common smut disease on maize (Zea mays) that can result in an economically significant reduction in yield (57, 58). Sexual development of the fungus is tightly interconnected with infection and involves several morphological transitions (3). The key transition for initial infection and for subsequent colonization of the plant is the production of the filamentous dikaryon. This cell type is established upon recognition of compatible haploid cells by pheromone exchange leading to the formation of conjugation tubes known as mating filaments that initially grow on the plant surface. Subsequently, these cells fuse to form a dikaryotic filament which is capable of invading host tissue. The mating filaments and the dikaryotic filaments prior to penetration are straight filaments that do not branch. Once in the plant, the fungus proliferates extensively to produce a large network of branched filaments. Mating and filamentation are controlled by conserved cyclic AMP/protein kinase A and mitogen-activated protein kinase signaling cascades; these control distinct stages of the disease process by largely unknown mechanisms (2, 4, 6, 13, 20, 21, 23, 24, 27, 44, 52). Recently, we showed that lipids and fatty acids induce filamentation in U. maydis (40). This response may be relevant to infection because the components of the protein kinase A and mitogen-activated protein kinase signaling networks are required for both the dimorphic transition and the response to lipids. Additionally, the morphological features of the lipid-induced filaments formed in vitro resembled those of the infectious dikaryon observed in planta.
U. maydis is an obligate biotrophic pathogen during the sexual phase of its life cycle. Infectious filaments initially invade epidermal cells and grow intracellularly surrounded by the intact host cell plasma membrane (70, 71). At this stage, early disease symptoms such as chlorosis and anthocyanin pigmentation are visible on infected maize plants. Later in development, filaments grow mostly intercellularly around cells of the vascular bundle (70). Following penetration and proliferation, the fungus induces tumors in which the cells exhibit extensive branching, hyphal fragmentation and the formation of melanized teliospores (i.e., sexual spores). The fungal cells in tumor tissue are embedded in thin-walled parenchymatous plant cells, which have been shown to lack plastids (14). To date, little is known about fungal genes that control or are required for development in the plant, and host signals that may contribute to pathogen development are not yet known. It is clear that the biotrophic fungal life style requires an intimate relationship with the plant because the host cells remain alive while metabolites are redirected to feed the pathogen. In this regard, U. maydis establishes long lasting interactions with maize, often without causing any visible damage to invaded cells and without provoking a defense response (3, 69). Therefore, it must have strategies to overcome resistance, either by masking its intrusion, suppressing host defense, and/or inducing specific host genes for the establishment of biotrophy. It has been shown, however, that drastic changes in transcript levels of maize genes related to metabolism and development occur during U. maydis infection (7). In general, it seems likely that sensing the nutritional state of the host environment during biotrophic growth is critical for disease development by U. maydis.
Our previous work indicated that lipids act as signals and as carbon sources to promote filamentous growth in culture for U. maydis (40). Given the relationship between filamentous growth and pathogenesis for U. maydis, and the abundance of lipids in plant tissue, it is possible that lipids are also important signals and/or carbon sources during maize infection. We have shown that U. maydis secretes lipase activity in culture to breakdown lipids, and assuming that this activity is expressed during infection (40), the released fatty acids could be further degraded via β-oxidation, a process by which fatty acids are broken down to acetyl coenzyme A (acetyl-CoA) by sequential removal of two carbon units in each oxidation cycle. A relationship between peroxisomal metabolic function and phytopathogenesis has been previously tested in the hemibiotrophic fungus Colletotrichum lagenarium (38). In this fungus, disruption of a gene for peroxisome biogenesis resulted in a defect in appressorium-mediated plant infection but the mutant retained the ability for invasive growth in planta. In addition, analysis of the transcriptome of the obligate biotrophic fungus Blumeria graminis at different stages in the life cycle revealed coordinate regulation of enzymes involved in primary metabolism, including lipid degradation enzymes (11). However, in this case, the fungus appears to use lipids stored in conidia to fuel colonization of host tissue via appressorium formation, and storage lipids are regenerated during growth in the host. These studies leave open the question of whether β-oxidation is required for successful infection by obligate fungal biotrophs. β-Oxidation could also contribute to the production of modified fatty acids that are known to influence development in fungi. For example, oleic acid and linoleic acid, and their derivatives, influence growth and spore formation in filamentous fungi (15, 16).
In this study, we made use of the fact that U. maydis is obligately biotrophic during the sexual stage of its life cycle but can also be cultured in the laboratory as a saprophyte. These properties allowed us to compare the contribution of peroxisomal β-oxidation to fungal morphogenesis and growth in culture with the requirement for this process during biotrophic infection. Specifically, we constructed and characterized U. maydis mutants lacking the mfe2 gene that encodes the multifunctional enzyme for the second and third steps in peroxisomal β-oxidation. We found that the mfe2 gene was required for the switch to filamentous growth on some but not all fatty acids and that it was needed for growth on long-chain fatty acids in culture. Inoculations of seedlings and developing ears with the mutants resulted in fewer tumors and delayed sporulation, indicating that mfe2 was necessary for full symptom development. Overall, these results suggest that lipids represent an important but not essential carbon source during biotrophic growth and raise the possibility that lipid utilization by U. maydis may influence additional aspects of infection such as signal perception or host defense.
MATERIALS AND METHODS
Growth conditions.
U. maydis strains were grown overnight at 30°C on both potato dextrose agar and potato dextrose broth (PDB; Difco) or complete medium (CM) (32). For selection of transformants, 250 μg of hygromycin B per ml was added to CM. To characterize the morphological response and to determine growth rate in lipids and fatty acids, 1 × 106 ml−1 of PDB-grown overnight cells were washed with sterile water and added to 5 ml of minimal medium (MM) (32) or spotted in a range of cell concentrations on MM agar supplemented with one of the following carbon sources (all added to a concentration of 1%): glucose, corn oil, or butyric, caproic, lauric, myristic, oleic, linoleic, erucic, arachidic, arachidonic, or jasmonic acid (all fatty acids were purchased from Sigma). The cells were grown at 30°C for 5 days with (liquid MM) or without (MM agar) shaking at 250 rpm. The growth of wild-type and mutant strains was determined by cell counts with a hemacytometer.
Strains, deletion constructs, and transformation procedures.
The DNA sequence of the mfe2 gene (Um00150) was originally obtained from the U. maydis genomic sequence at Broad Institute (http://www.broad.mit.edu/annotation/fungi/ustilago_maydis/). Additional sequence information (Um10038) came from the Munich Information Center for Protein Sequences (MIPS) U. maydis genome annotation project (http://mips.gsf.de/genre/proj/ustilago/). A PCR overlap strategy was used to generate the Δmfe2::HygBr deletion construct (18). The Δmfe2::HygBr was designed to delete the entire open reading frame of the mfe2 gene. From genomic DNA, a 655-bp 5′ flanking region and a 550-bp 3′ flanking region were amplified using primers MFE2P1 (5′-AGTTTCGAGTCGGTGCGT-3′) and MFE2P2 (5′-AACTGTGCTTCAATCGCTGCGGTATGCGGCTGTGAGTTGA-3′) and primers MFE2P5 (5′-TAGCACACGACTCACATCTGCGAGTGCATGGTGGTGAGAT-3′) and MFE2P6 (5′-TGAGCGTTGCAATCGTGA-3′), respectively. The 2.7-kb hygromycin resistance marker was amplified using primers MFE2P3 (5′-TCAACTCACAGCCGCATACCGCAGCGATTGAAGCACAGTT-3′) and MFE2P4 (5′-ATCTCACCACCATGCACTCGCAGATGTGAGTCGTGTGCTA-3′) from the plasmid DNA pIC19RHL. The three fragments were combined by an overlapping PCR using primers MFE2P1n and MFE2P6n. The 4-kb overlap PCR product generated the Δmfe2::HygBr construct, which was cloned into pCR2.1 (Invitrogen). The plasmid containing the deletion construct was transformed into Escherichia coli strain DH10B (Bethesda Research Laboratories).
The deletion strains a1b1 Δmfe2::HygBr and a2b2 Δmfe2::HygBr were generated by biolistic transformation (78) of a1b1 (521) and a2b2 (518) strains (31). Transformants were screened by colony PCR using a U. maydis-specific primer outside the construct MFE2CR (5′ to 3′; TCT CGC ACC AAT CAA TCC TG) and hygB-specific primer HYGBL (5′-ATC AGT TCG GAG ACG CTG-3′). Gene deletion was also confirmed by DNA blot analysis using genomic DNA and blots prepared and hybridized by standard methods (63). Two independent mutants in each strain were analyzed.
RNA isolation and northern analysis.
Cells were grown overnight in 5 ml MM supplemented with glucose (1%) and transferred to MM supplemented with either caproic, oleic, or linoleic acid (all added to 1%) and grown at 30°C at 250 rpm for 6 h. RNA was isolated as described previously (64). RNA blot preparation and hybridization was performed using standard methods (63). PCR was used to amplify the 150-bp DNA fragment as a hybridization probe using primers MFE2NP1 (5′-AGAGCACCGTCTTCATTCG-3′) and MFE2NP2 (5′-TGTGAAGCGCACCTTGATG-3′). The probe was labeled with 32P by random priming (ReadiPrime II Oligolabeling kit; Amersham Pharmacia Biotech).
Sequence analysis.
Gene prediction, protein alignments, and sequence analysis were done using the programs BLAST (1), CLUSTAL W (77), and Pfam (8).
Microscopy and staining procedures.
Fluorescent brightener 28 calcofluor white (F3543; Sigma) was used to visualize cell walls; and 1 μl of a 20 μg/ml solution was added directly to 5 μl of cell culture spotted on a slide. Nile red (Sigma N3013) was used to visualize lipid bodies by adding 1 μl of a 0.1 mg/ml solution in 100% acetone directly to 5 μl of cell culture, incubated for 5 min, and observed using a fluorescein isothiocyanate filter. Nile red stains intracellular lipids that localize in lipid bodies (39). Fungal proliferation in a plant tissue was observed in epidermal peels generated from maize leaves at 2, 4, and 7 days postinoculation. The thin layers of plant cells were placed on a 30-μl drop of water with 3 μl of fluorescent brightener 28 calcofluor white (F3543; Sigma) for microscopic observation. Cross sections of tumor tissue collected from infected mature plants 14 and 20 days postinoculation were generated using a razor blade. The cross sections were placed on a 30-μl drop of water with 3 μl of fluorescent brightener 28 calcofluor white (F3543; Sigma) for microscopic analysis. Cells were observed using a Zeiss Axioplan 2 fluorescence microscope with differential interference contrast (DIC) optics or UV fluorescence to observe cells stained with calcofluor or Nile red. Images were captured with a charge-coupled-device camera and processed with Northern Eclipse imaging software and Adobe Photoshop 7.
For electron microscopy, cells were prefixed by the addition of glutaraldehyde (2.5%) to cultures grown for 18 h either on glucose (1%) or oleic acid (1%) with shaking at 250 rpm. The cells were harvested by centrifugation for 10 min at 16,100 × g at 20°C, resuspended in 50 mM phosphate buffer (pH 6.8) containing 3% glutaraldehyde to an optical density at 600 nm of 10, and fixed for 24 h at room temperature. For specific lipid staining, the cells were postfixed with 2% OsO4 in 200 mM imidazole buffer (pH 7.5) for 1 h. After washing with 100 mM imidazole buffer (pH 7.5), the cells were dehydrated in a graded ethanol series in the following order: 50, 70, 90, and 100% (vol/vol). For transmission electron microscopy (TEM), the cells were embedded in Spurs resin, and 70-nm-thick sections were cut with a Leica Ultracut E ultramicrotome and stained with 2% uranyl acetate for 14 min and lead citrate for 7 min. Sections were mounted on 200-mesh grids and examined with a Hitachi H7600 TEM. A random sample of cells was examined in three separate cultures from each type of medium.
Lipid extraction and fatty acid analysis.
Cells were grown in 50 ml MM supplemented either with glucose (1%) or oleic acid (1%) at 30°C at 250 rpm for 5 days. The cells were harvested by centrifugation and washed twice with hexane (H9379; Sigma). To extract the intracellular lipids, the cells were sonicated, 2 ml methanolic KOH was added to saponify lipids for 2 h at 80°C, and then cells were washed by the addition of 1 ml water and 2 ml hexane. Four hundred microliters of concentrated HCl and 2 ml hexane were added to the bottom phase to extract free fatty acids. Heptadecanoic acid (C17:0) (H3500; Sigma) was added to the extracted lipids as an internal standard. Free fatty acids were converted into fatty acid methyl esters by adding diazomethanol and incubating them at room temperature for 4 h. The fatty acid methyl esters were analyzed by gas chromatography mass spectrometry (GC/MS) with an Agilent Technologies 5975 Inert XL MS Detector with 6890N GC and 7683 B Series autosampler equipped with a capillary GC column Agilent HP-5MS 5% phenyl methyl siloxane.
Mating and virulence assays.
Strains were tested for their ability to mate by the production of white aerial hyphae during mating reactions on charcoal-containing CM (32). For virulence assays, mating cultures of 1 × 107 cells ml−1 (grown on PDB overnight at 30°C with shaking at 250 rpm) generated by crossing the strains in the following combinations was used to infect maize plants: 521 (a1b1) × 518 (a2b2), 521 (a1b1) × a2b2 Δmfe2::HygBr, 518 (a2b2) × a1b1 Δmfe2::HygBr, and a1b1 Δmfe2::HygBr × a2b2 Δmfe2::HygBr. For seedling infections, 1-week-old maize plants (Golden Bantam) were inoculated by injecting approximately 100 μl of mating cultures per plant. After 14 days, plants were scored for disease symptoms using the following ratings: 1, chlorosis and pigment production; 2, small leaf tumors; 3, small stem tumors; 4, large stem tumors; 5, plant death. Approximately 100 plants for each combination of strains were scored for disease symptoms. For mature plant infections, 2- to 3-month-old maize plants were inoculated by injecting approximately 2 ml of mating cultures into the silk channels of developing cobs. The infections were repeated four times. The production of teliospores within tumors was examined after 14 and 20 days.
Accession numbers.
Sequence data from this article can be found in the EMBL Nucleotide Sequence Submission (EMBL), http://www.ebi.ac.uk, and GenBank, National Center for Biotechnology Information (GenBank), http://www.ncbi.nlm.nih.gov, data libraries under accession number XP_756297.
RESULTS
Identification of genes encoding peroxisomal β-oxidation enzymes.
As an initial step to characterize the role of β-oxidation in U. maydis pathogenesis, the sequences of the enzymes involved in the process in Saccharomyces cerevisiae were used to identify homologs in the U. maydis genomic sequence recently completed at the Broad Institute (with annotation at MIPS). The first committed step in peroxisomal β-oxidation is catalyzed by acyl-CoA oxidase, a unique marker enzyme of nonmitochondrial β-oxidation in eukaryotic cells (42). There are five U. maydis genes (Um04324, Um02208, Um01966, Um02028, and Um04833) predicted to encode acyl-CoA oxidases (homologs of the yeast protein Pox1/Fox1). The second and third steps in β-oxidation are catalyzed by a multifunctional enzyme that is encoded by FOX2 (CAA82079) in S. cerevisiae (30). U. maydis has one homolog encoded by a gene designated mfe2 (Um10038; multifunctional enzyme type 2). The Mfe2 protein shows 47% identity and 63% similarity to the yeast Fox2 protein over 721 amino acids. The fourth enzymatic reaction is catalyzed by 3-ketoacyl CoA thiolase, and there are three homologs (Um03571, Um01090, and Um02715) with similarity to the yeast protein Fox3. Because a single gene (mfe2) was found to encode the second and third steps, this gene was chosen for subsequent deletion to generate mutants that would be unable to utilize fatty acids as a carbon source. Such mutants would allow an investigation of whether lipid metabolism had an influence on fatty acid-induced filamentation and virulence in U. maydis.
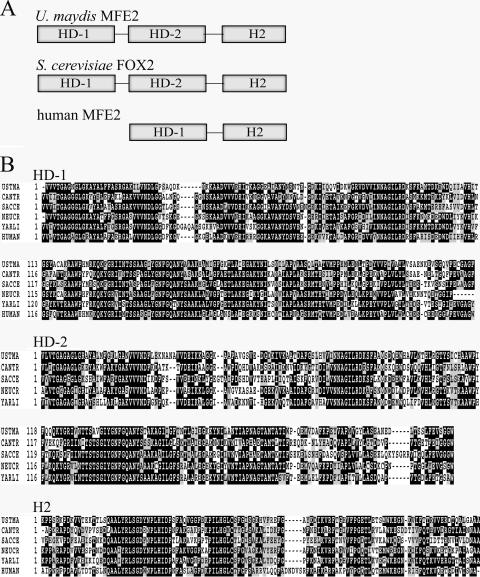
The mfe2 gene encoded a predicted polypeptide of 911 amino acids with a duplicated region for the two dehydrogenase domains in the first half of the protein (PF00106/IPR002198; amino acid regions 19 to 249 and 326 to 499) (Fig. 1). A separate hydratase domain (PF01575/IPR002539) was present in the C-terminal region. It is possible that the dehydrogenase domains may contribute to different substrate specificities; for example, the first domain is most active with long- and medium-chain substrates, and the second domain with short-chain substrates in S. cerevisiae (60). The C-terminal domain encoding the 2-enoyl-CoA hydratase 2 is common to all multifunctional enzymes of the Mfe2 type (Fig. 1). The mammalian peroxisomal Mfe2 enzyme contains only one dehydrogenase domain with broad substrate specificity (both long- and short-chain substrates) (56) (Fig. 1A). At least three types of peroxisomal targeting sequences (PTSs) direct proteins to peroxisomes in yeast, including PTS1 and PTS2 and a third type that has not been well characterized (19, 59). For example, some of the acyl-CoA oxidases in Candida albicans or S. cerevisiae do not possess PTS1 or PTS2 and may be targeted to peroxisomes by either of two internal, redundant sequences (37, 67). Neither PTS1 nor PTS2 was found in the U. maydis Mfe2 sequence, and the enzyme may possess the third type of PTS. In addition to the high amino acid sequence identity of Mfe2 to the yeast Fox2 protein, BLAST analysis confirmed sequence similarity to other characterized peroxisomal multifunctional enzymes from the following fungi: Candida tropicalis (P22414, 47% identity) (54), Neurospora crassa (CAA56355, 54% identity) (22), Yarrowia lipolytica (AAF82684, 55% identity) (68), and Glomus mosseae (Q9UVH9, 53% identity) (62) (Fig. 1B).
FIG. 1.
Organization of the peroxisomal multifunctional enzymes (type 2). (A) Organization of the catalytic domains of U. maydis, S. cerevisiae, and human peroxisomal Mfe2 enzymes. (B) Sequence alignment of the conserved catalytic domains from the U. maydis Mfe2 protein with fungal (C. tropicalis [CANTR, P22414], S. cerevisiae [SACCE, CAA82079], N. crassa [NEUCR, CAA56355], and Y. lipolytica [YARLI, AAF82684]), and human (P51659) homologs. HD, (3R)-hydroxyacyl-CoA dehydrogenase domain; H2, 2-enoyl-CoA hydratase 2 domain.
Induction of mfe2 gene expression by fatty acids.
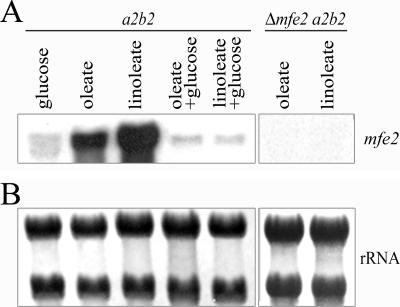
Fatty acids induce expression of the genes/enzymes involved in β-oxidation and transcription of the corresponding genes is subject to glucose repression in yeast (43, 49, 73, 87). We established that the mfe2 gene fits this pattern because RNA blot analysis revealed that transcript levels were relatively high upon growth in medium with oleic acid (C18:1) or linoleic acid (C18:2) as the sole carbon source (Fig. 2). Transcript levels were lower in cells grown on these fatty acids in the presence of glucose and in cells grown on glucose as the sole carbon source. These results indicate that mfe2 transcription is influenced by carbon source, as expected for a β-oxidation function.
FIG. 2.
RNA blot analysis of mfe2 transcript levels in the presence of fatty acids. (A) Total RNA was isolated from wild-type (a2b2) cells grown in minimal medium containing either glucose, fatty acid, or fatty acid and glucose. Total RNA was also isolated from the Δmfe2 a2b2 cells grown in minimal medium containing fatty acids. The RNA blot was hybridized with a probe for the mfe2 gene. (B) The RNA blot stained with 0.04% methylene blue to show total RNA loading.
Targeted deletion of the mfe2 gene.
To investigate the role of β-oxidation during the growth of U. maydis in culture and in planta, we performed a targeted gene deletion by replacing the entire open reading frame of mfe2 with a 3.8-kb gene cassette conferring resistance to the antibiotic hygromycin B. The deletion vector was introduced into two U. maydis wild-type strains of opposite mating type (a1b1 and a2b2) to allow subsequent analysis of mating and virulence. Deletion of the gene was confirmed by colony PCR and Southern blot analysis (not shown) and two independent mutants in each strain were used for subsequent experiments. RNA blot analysis revealed the absence of the mfe2 transcript in the mutants, thereby confirming complete gene inactivation (Fig. 2).
Growth and filamentation on LCFA.
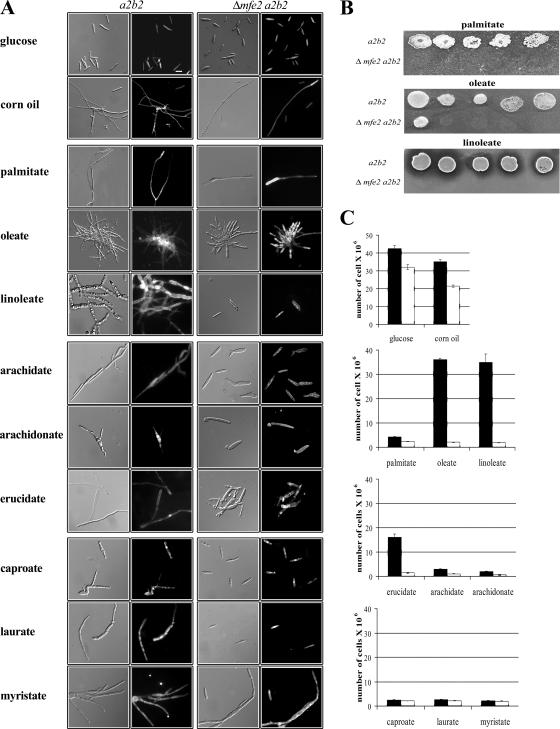
We initially characterized the ability of the mfe2 mutants to undergo a morphological transition from budding to filamentous growth in the presence of lipids and fatty acids. Palmitic (C16:0), oleic (C18:1), and linoleic (C18:2) acids were chosen for this study because our previous analysis demonstrated that wild-type strains respond to these fatty acids (40) and these are also the most abundant fatty acids present in maize plants (90, 91). As expected, the wild-type strains grew as filaments in the presence of all of the long-chain fatty acids (LCFA) tested and in the presence of corn oil (Fig. 3A). The mutant strains responded to corn oil, palmitic acid, and oleic acid by switching from budding to filamentous growth thus indicating that the mfe2 gene was not generally required for fatty acid-induced filamentation (Fig. 3A). This observation suggests that it is possible to separate the utilization of fatty acids as a carbon source from their function as signaling molecules in the filamentous response. However, the mutants did not respond morphologically to linoleic acid, thus raising the possibility that specific fatty acids may contribute to the production of signals that influence filamentous growth.
FIG. 3.
Morphology and growth of mfe2 mutant strains on fatty acids differing in carbon chain length and saturation state. (A) Cellular morphology of the wild-type (a2b2) and mutant (Δmfe2 a2b2) strains in response to glucose, lipids (corn oil), LCFA (palmitic, oleic, and linoleic), VLCFA (erucic, arachidic, and arachidonic), SCFA (caproic), and MCFA (lauric and myristic). The cells were visualized by DIC optics (left) and by epifluorescence after staining cell walls with calcofluor (right). (B) The ability of the wild-type and mutant strains to grow on fatty acid-containing agar medium. The cells were spotted in decreasing concentration from 106 to 102. All of the agar plates contain tergitol to facilitate the solubility of the fatty acids. (C) Total number of the wild-type (a2b2, black bars) and mutant (Δmfe2 a2b2, white bars) cells in culture supplemented with glucose, corn oil, and fatty acids as a sole carbon source. The bars represent the average number of cells from three independent experiments.
In addition to the general filamentation response, differences in the morphologies of the wild-type cells were observed in the presence of different LCFA (Fig. 3A). Wild-type cells grown in oleic acid developed large rounded central cells with long branched filaments emerging from the center. Interestingly, the oleic acid-grown cultures of the wild-type cells were also pigmented with a dark brown to black color (data not shown). Wild-type cells grown on linoleic acid developed an extensive network of robust and branched filaments with many rounded cells. Some of these cells had lobed ends and resembled the filaments observed in the early stages of sporulation in plant tissue (3). In palmitic acid, the wild-type cells grew as long straight hyphae that resembled the straight mating filaments and the infectious dikaryotic filaments that form before and after the fusion of compatible strains, respectively. Furthermore, these hyphae produced very few branches, a phenotype observed only on palmitic acid. Overall, these results support the idea that long-chain, unsaturated fatty acids may be important in promoting branching in wild-type cells and thus may contribute to extensive proliferation during growth in plant tissue. Our findings in culture suggest that linoleic acid may be particularly important in this role.
The mfe2 mutants were also tested for their ability to utilize fatty acids by growing the cells on fatty acid-containing agar medium supplemented with LCFA (palmitic, oleic, and linoleic acids) and in a liquid medium supplemented with triacylglycerols (corn oil) and LCFA (palmitic, oleic, and linoleic acids) as the sole carbon source (Fig. 3B and C). The mutants were able to grow on corn oil (Fig. 3C). This was not surprising because the triacylglycerols consist of fatty acids esterified to a glycerol backbone, and U. maydis is known to be able to use glycerol as a carbon source (40). The wild-type strains grew well on corn oil, oleic acid, and linoleic acid compared to growth on glucose (Fig. 3C). The mutant strains were unable to efficiently utilize oleic and linoleic acids as a carbon source, although a low level of residual growth was observed for both of these fatty acids when grown in liquid medium (Fig. 3C). The mutants grew poorly on palmitic acid, although the growth of the wild-type strain was also reduced, suggesting that U. maydis generally does not efficiently utilize this fatty acid. In addition, the mutant strains failed to grow on all of the LCFA tested when spotted in a range of cell concentrations on fatty acid-containing agar plates (Fig. 3B). In contrast, the wild-type strain grew well on corn oil and oleic and linoleic acids compared to growth on glucose (Fig. 3C). Overall, we conclude that loss of mfe2 results in growth defects on LCFA, as expected for strains with a defect in β-oxidation.
Growth and filamentation on VLCFA.
Fungi require peroxisomal β-oxidation to break down very long chain fatty acids (VLCFA), and we therefore investigated the ability of these fatty acids to induce filamentation in mfe2 mutants. The wild-type and mutant strains were inoculated into medium with one of the following fatty acids as a sole carbon source: arachidic (C20:0), arachidonic (C20:4), and erucic (C22:1). All of these VLCFA induced filamentous growth in the wild-type strains, although the filaments in arachidonic acid had a slightly different appearance with primarily short branching cells (Fig. 3A). In contrast, none of the VLCFA induced filamentation in the mfe2 mutants. These mutants failed to grow on VLCFA, and the wild-type strain also showed poor growth on arachidic and arachidonic acids but not erucic acid (Fig. 3C). In addition, jasmonic acid was also tested for the ability to induce filamentation; however, it completely abolished the growth of both the wild-type and mutant cells (data not shown). We also noted that both the wild-type and mutant strains produced extracellular needle-like crystal structures in the medium with erucic acid (data not shown). These crystals could be glycolipids because U. maydis is known to produce extracellular glycolipids called ustilipids (10, 29, 46) that are visible as needle-like precipitates. Interestingly, similar long needle-like crystals were also observed when wild-type strains were grown in the medium supplemented with both oleic and linoleic acids but not in the medium containing either one of these fatty acids alone (data not shown). Taken together, these observations suggest that the fungus may need peroxisomal β-oxidation to break down VLCFA to produce shorter fatty acyl chains or to contribute to the production of putative modified fatty acids that might promote filamentous growth.
Growth and filamentation on SCFA and MCFA.
The ability of short-chain fatty acids (SCFA), caproic acid (C6:0), or medium-chain fatty acids (MCFA), lauric (C12:0) and myristic (C14:0) acids to induce filamentation was also examined (Fig. 3A). The wild-type strain responded by growing as short distorted branched cells in all of the SCFA and in lauric acid but produced long branched filaments in myristic acid. Intriguingly, the mfe2 mutants did not display a filamentous response to any of these fatty acids, except myristic acid. We also explored the ability of the mfe2 mutants to grow on SCFA and MCFA and found that these carbon sources supported only a limited growth for both the wild-type and mutant strains (Fig. 3C). SCFA and MCFA have previously been shown to inhibit growth in some fungi (50). Growth was not completely abolished but was limited to approximately four cell doublings. These results suggest the existence of another utilization pathway (e.g., mitochondrial β-oxidation) or residual growth on stored lipids.
Cellular lipid accumulation is influenced by loss of peroxisomal β-oxidation.
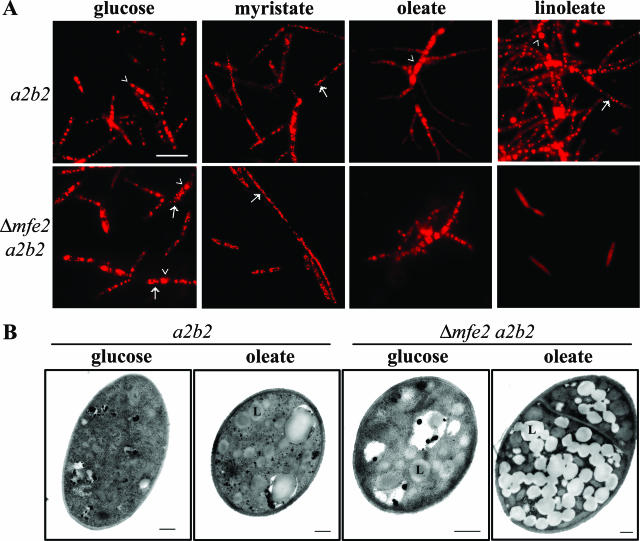
The loss of Mfe2 function clearly alters the growth and filamentation response of U. maydis to different fatty acids. A defect in the homologous gene, FOX2, in S. cerevisiae mutants also alters lipid body production. We therefore used Nile red to observe and compare the accumulation of lipid bodies in the wild-type and mutant cells grown on glucose as well as on inducing (myristic, oleic) or noninducing (linoleic) fatty acids. The yeast-like cells grown on glucose produced 4 to 6 large lipid bodies in the wild-type cells and 2 to 4 large and some small lipid bodies in the mutant cells (Fig. 4A). Overall, the accumulation pattern of the lipid bodies appeared to be unaffected in the mfe2 mutants grown on glucose. However, the lipid bodies varied in size and number in the fatty acid-induced filamentous cells depending on the fatty acid present in the growth medium. Specifically, the lipid bodies were very small and numerous in both wild-type and mutant cells on myristic acid (Fig. 4A). This result suggests that both cell types accumulate and store myristic acid in lipid bodies that have structural differences compared with those formed in glucose-grown cells. The oleic acid-induced filaments of wild-type cells contained large lipid bodies in the central cells and only diffuse fluorescence in the filaments branching from these cells (Fig. 4A). In contrast, the lipid bodies in the mutant cells on oleic acid were dispersed and visible as small intensely stained droplets throughout the short branched cells (Fig. 4A). A more striking difference was observed for the cells grown on linoleic acid. In this case, the wild-type filaments were filled with numerous large and small lipid bodies throughout their entire length, but the mutant cells did not produce lipid bodies and did not change their morphology (Fig. 4A). Only a diffuse fluorescence was observed within the yeast-like cells of the mutant (Fig. 4A).
FIG. 4.
Intracellular lipids in U. maydis. (A) Cellular lipid accumulation in U. maydis wild-type and mfe2 mutant strains grown on glucose and various fatty acids. The wild-type and mfe2 mutant strains were grown in minimal medium supplemented with either glucose, myristic (C14:0), oleic (C18:1), or linoleic acid (C18:2) as a sole carbon source. The internal lipids accumulated in lipid bodies were stained using the lipid-specific fluorescent dye Nile red and visualized using epifluorescence. The fungal cells produced large (arrowhead) to small (arrow) lipid bodies that varied in number depending on carbon source. Bar, 10 μm. (B) An abundance of lipid bodies produced in the mfe2 mutant strain grown on oleic acid as a sole carbon source. TEM observation of lipid bodies in wild-type cells (a2b2) and mfe2 mutant (Δmfe2 a2b2) cells grown on glucose and oleic acid. Cells were grown on glucose medium overnight and then transferred into oleic acid medium. After 18 h, the oleic acid-grown cells were fixed and processed for TEM. Bar, 500 nm. L, lipid body.
The ability of cells to respond to fatty acids (e.g., oleic acid) by forming filaments in comparison to the yeast-like cells found on glucose is particularly interesting and may be relevant to the filamentous growth observed in planta. We therefore performed a more detailed comparison of the lipid accumulation in the different morphological types by electron microscopy and by chemical analysis. We examined the mfe2 mutants by TEM to compare the size and numbers of the lipid bodies with those in wild-type strains (Fig. 4B). We found that very few lipid bodies were observed in the yeast-like wild-type cells grown on glucose, while the mfe2 mutant cells appeared to produce more and larger lipid bodies in this medium (Fig. 4B). In contrast, the lipid bodies in the oleic acid-induced wild-type filaments were more easily identified and were often found in clusters (Fig. 4B). There was a striking difference in the accumulation of the lipid bodies in the mfe2 mutant strain grown on oleic acid compared to the wild-type strain (Fig. 4B). The numerous lipid bodies almost completely filled the mutant cells. Therefore, these cells appeared to accumulate but not metabolize the exogenous fatty acids from the medium, as expected for cells defective in β-oxidation.
Total internal lipids were also extracted from wild-type and mfe2 mutant cells grown either on glucose or oleic acid to examine whether the mfe2 deletion alters intracellular lipid composition and to evaluate potential changes in fatty acid composition during fatty acid-induced filamentation. The extracted internal lipids were converted to methyl esters and analyzed using gas chromatography to determine fatty acid species in the different cell types. This analysis revealed differences in the total abundance of fatty acids between the wild-type and the mfe2 mutant cellular lipids (Table 1). Specifically, more linoleic (C18:2) than oleic (C18:1) acid was found in wild-type and mutant yeast-like cells grown on glucose. Oleic acid was the predominant fatty acid in filamentous cells from oleic acid medium, accounting for 79% of total fatty acids in the wild-type and 90% in the mfe2 mutant cells. Strikingly, linoleic acid comprised only 9% of total fatty acids in the oleic acid-grown mutant cells. The mutant accumulated oleic acid in higher levels relative to linoleic acid than did the wild-type strain. The ratio between oleic and linoleic acid in the mutant cells was 10:1 compared to 4:1 in the wild-type cells. This finding correlates with the structural data showing an excessive accumulation of lipid bodies in mfe2 mutants grown on oleic acid (Fig. 4B). One possibility is that the mutant is unable to breakdown oleic acid available in the environment but is still able to accumulate the fatty acid to excessive amounts compared to the wild-type cells. The yeast-like cells grown on glucose contained more linoleic acid than the filamentous cells grown on oleic acid, which contained higher levels of endogenous oleic acid. Moreover, both glucose-grown wild-type and mutant strains, which exhibit yeast-like morphology, accumulated more saturated palmitic acid (C16:0) than oleic acid-grown cells, accounting for 23% of total lipids in the wild-type cells and 17% in the mutant cells. Palmitic acid was found only in trace amounts in the oleic acid-induced filamentous cells. Taken together, these comparisons indicate that the mfe2 mutants are significantly different from wild-type cells in terms of their accumulation of lipid bodies and their fatty acid composition when grown on oleic acid. These differences and the growth defects of the mutant on fatty acids raised the possibility that the mutants would have reduced growth during infection. We addressed this possibility in inoculation experiments with both vegetative tissue (seedlings) and floral tissue (developing ears), as described in the following sections.
TABLE 1.
Fatty acid profiles of total internal lipids extracted from U. maydis wild-type (a2b2) and mfe2 mutant (Δmfe2 a2b2) strains grown either on glucose or oleic acida
| Fatty acidd | Result (%b ± SD) for strain and carbon source
|
|||
|---|---|---|---|---|
|
a2b2
|
Δmfe2 a2b2
|
|||
| Glucose | Oleic acid | Glucose | Oleic acid | |
| C14:0 | 1 ± 0.2 | NDc | tre | ND |
| C16:0 | 23 ± 0.5 | tr | 17 ± 2.1 | tr |
| C16:1 | 1 ± 0.0 | 1 ± 0.5 | 1 ± 0.5 | 1 ± 0.0 |
| C18:0 | 5 ± 1.3 | tr | 4 ± 0.5 | tr |
| C18:1 | 29 ± 2.4 | 79 ± 3.3 | 20 ± 3.8 | 90 ± 0.2 |
| C18:2 | 42 ± 3.0 | 20 ± 5.0 | 57 ± 4.3 | 9 ± 1.7 |
| C20:0 | tr | tr | tr | ND |
The table shows data from three independent experiments.
Percentage of total fatty acids.
ND, not detected.
Internal lipids were extracted from cells grown in minimal medium supplemented with the appropriate carbon source for 20 h. Fatty acids were analyzed by gas chromatography.
tr, found in trace amounts.
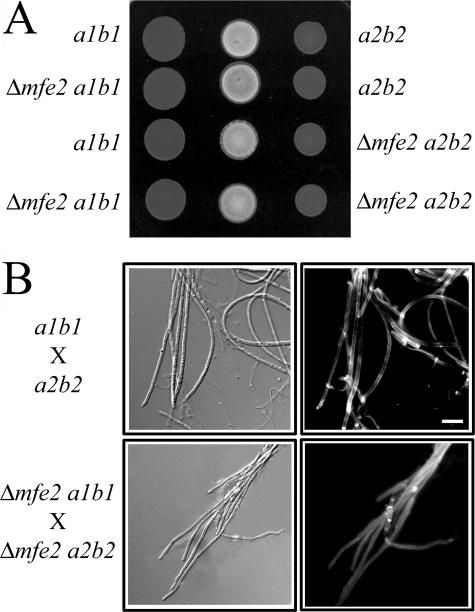
Mfe2 is not required for the production of mating filaments.
U. maydis must mate to produce the filaments required to infect plant tissue. The mating filaments are easily visualized on charcoal-containing agar plates where they generate white, fuzzy colonies in contrast to the smooth colonies produced by haploid yeast-like strains (4). The mfe2 mutants produced white, aerial hyphae during mating, thus indicating a positive mating reaction (Fig. 5A). The mating filaments were scraped from the surface of the plate and examined microscopically. The mating filaments produced from the mixture of compatible mfe2 mutant strains did not exhibit any differences in cellular morphology with respect to the wild-type strains (Fig. 5B). The filamentous morphology resembled that of the wild type in that they were straight with collapsed hyphal compartments. The cells at the growing tips were elongated with visible chitin accumulation (Fig. 5B). Overall, mating is unaffected in mfe2 mutants, suggesting that β-oxidation does not play an essential role in the formation of the infectious cell type.
FIG. 5.
Mating filaments produced by compatible mfe2 mutant strains during mating. (A) Mating test of wild-type (a1b1 and a2b2) and mfe2 mutant strains. Each strain was spotted on charcoal-containing medium, and the compatible strains were mixed in the center of the plate to assess the mating reaction. The positive mating reaction is represented by the production of white filaments that are visible on the dark medium. (B) Dikaryotic filaments formed by a cross of the wild-type and mfe2 mutant strains on charcoal-containing medium after 48 h. Both wild-type and mutant strains produced unbranched mating and dikaryotic filaments with collapsed sections of hyphal cells. The images were captured using DIC optics (left panel) or epifluorescence (right panel) to visualize calcofluor-stained cell walls. Bar, 10 μm.
Loss of mfe2 results in attenuation of virulence during plant infection.
The inoculation of corn seedlings with compatible wild-type and mfe2 mutant strains revealed that the mutants were clearly attenuated for virulence, although mild disease symptoms were observed (Table 2). The mutants were still able to induce tumors on leaves and basal parts of the plant stems and to produce teliospores. However, only 27% of the plants infected with the mixture of compatible mfe2 mutant strains developed tumors compared to 88% of the plants infected with the compatible wild-type strains (Table 2). A large proportion (73%) of the plants infected with the mutant strains developed only chlorosis and anthocyanin pigmentation. We also tried to remediate the virulence defect by including 1.5% or 3% glucose with the inoculum as a way to potentially bypass nutritional defects in the mutant strains. This treatment did not change the outcome in terms of the severity of disease symptoms (data not shown). However, it is unclear whether the glucose would persist for a sufficient time and in the proper location to support all stages of fungal growth during infection. The teliospores produced in tumors of the plants infected with the mfe2 mutants were also tested for their ability to germinate and no obvious difference was found compared to spores from the wild-type inoculations (data not shown). These data suggest that β-oxidation is not essential for U. maydis to complete the life cycle in planta, but it is required for robust growth of the fungus in the host and the development of full disease symptoms.
TABLE 2.
Pathogenicity of mfe2 mutantsa
| Cross or strain | No. of plants:
|
% of plants with tumors | Disease scoreb | ||
|---|---|---|---|---|---|
| Producing anthocyanin | With tumors | Infected (total) | |||
| a1b1 × a2b2 | 12 | 87 | 99 | 88 | 3.7 |
| a1b1 × Δmfe2 a2b2 | 16 | 95 | 106 | 90 | 3.4 |
| Δmfe2 a1b1 × a2b2 | 10 | 82 | 92 | 89 | 3.6 |
| Δmfe2 a1b1 × Δmfe2 a2b2 | 75 | 28 | 103 | 27 | 1.2 |
Table shows combined data from four independent experiments.
The disease score is calculated as the sum of disease symptom ratings divided by the total number of infected plants scored for symptoms.
mfe2 mutant strains do not proliferate extensively in planta.
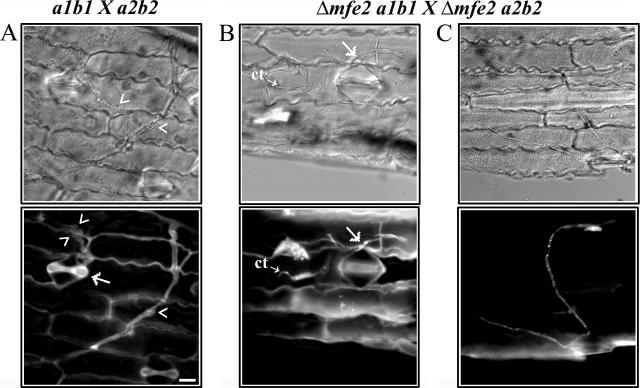
Given the virulence defect in mfe2 mutants, we investigated the importance of the mfe2 gene during the initial penetration and subsequent proliferation of the infectious filaments in planta. Epidermal peels from maize leaves infected with the compatible wild-type and mfe2 mutant strains were collected at 1, 4, and 7 days postinoculation and stained with calcofluor for visualization of fungal cells. Straight mating filaments and the infectious dikaryotic filaments that result from compatible mating reactions were observed on the surface of the maize leaves of both wild-type and mutant infections 1 day after inoculation. Many of the wild-type, dikaryotic filaments had entered the plant epidermis, mostly through stomata. In contrast, the dikaryotic filaments of the mutant had not yet penetrated the epidermal layer at this stage. After 4 days postinoculation, many of the mutant filaments had penetrated the plant tissue and had started to grow inside the plant tissue; however, no obvious branching of the filaments was visible. At this time, the wild-type filaments showed extensive growth within the plant cells, and many branched hyphal growing tips were observed (Fig. 6A). After 7 days postinoculation, a large network of the mutant filaments was observed within the plant cells. However, no obvious branching of the invading filaments was observed (Fig. 6C). Many yeast-like cells of the mutant strains were still found on the epidermal surface, often clustered around stomata (Fig. 6B). In contrast, the wild-type infection resulted in large networks of filamentous cells that were extensively branched. These results suggest that deletion of mfe2 reduced the ability of the fungus to produce the highly branched filaments that are crucial for fungal proliferation in host tissue.
FIG. 6.
Hyphal morphology in planta. (A) Wild-type filaments growing within plant tissue 7 days after inoculation with compatible wild-type strains (a1b1 × a2b2). The filaments branched (arrowheads) and could penetrate epidermal cells through a stoma (arrow). The tip of the penetrating hypha is out of the focal plane. (B) Yeast-like cells of compatible mfe2 mutant strains on the epidermal surface of a maize leaf. Some of the cells were elongated and started to produce conjugation tubes (ct), possibly in response to a mating partner. The cells often clustered around stomata, and once a dikaryotic filament was formed, it sometimes penetrated through the stomata (arrow). (C) mfe2 filament growing within plant tissue. The mutant filaments were often observed on the epidermal surface or within plant tissue without branching, exhibiting typical straight mating-like hyphal morphology. Epidermal peels from maize leaves were examined using DIC (top panel) and epifluorescence (bottom panel) to visualize the calcofluor-stained cells. Bar, 10 μm.
Deletion of mfe2 delays teliospore development within tumor tissue.
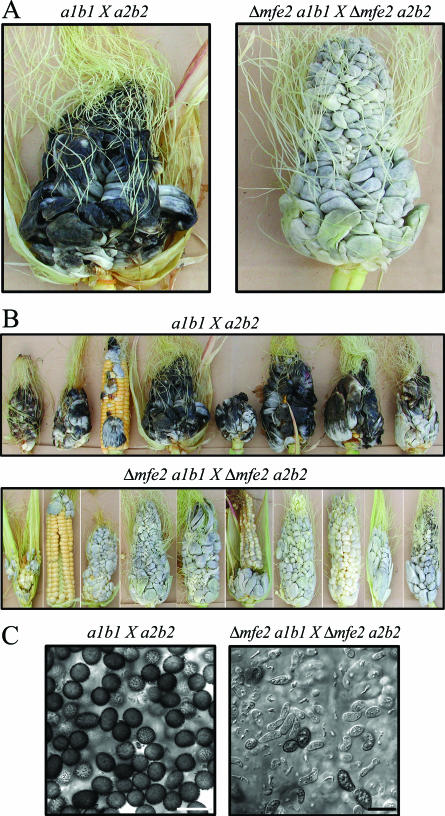
U. maydis infects any aboveground part of the plant, and symptoms are particularly dramatic in developing ears of mature plants. To examine the virulence defect seen in young seedlings more closely, we infected developing ears of 2- to 3-month-old plants with compatible wild-type and mutant strains. The plants infected with the mutants showed a delay in symptom formation that was consistent with the defect seen in infected seedlings (Fig. 7). The individual kernels of cobs infected with wild-type strains had developed into large black tumors containing an abundance of mature, melanized teliospores by 14 days after inoculation (a total of 13 cobs with tumors were collected in three independent experiments) (Fig. 7A, B, and C). In contrast, the cobs infected with the mutant strains were white and cross sections of these tumors indicated that they contained only immature spores (a total of 12 cobs with tumors were collected in three independent experiments) (Fig. 7A, B, and C). Specifically, the fungus within the tumors was found in several of the stages of development that occur prior to maturation of teliospores (3). These stages included bloated hyphae with lobed hyphal tips embedded within a mucilaginous matrix (appearing approximately 7 to 8 days postinoculation in a wild-type cross), fragmentation of sporogenic hyphae producing individual fragments containing cells in the process of rounding (appearing approximately 9 days postinoculation in a wild-type cross), cells with different morphologies that are in the process of forming teliospores, and immature teliospores which are not yet melanized (appearing approximately 12 days postinoculation in a wild-type cross) (Fig. 7C). The development of teliospores by the mutant strains was also examined at 20 days after inoculation to assess whether spores would eventually form at wild-type levels. A high proportion of melanized teliospores was observed within the 20-day-old tumor tissue, indicating that the mutants were delayed in their development compared to the wild-type strains. These observations support the conclusion that the loss of mfe2 influences the pathogenic development of U. maydis.
FIG. 7.
Teliospore production of mfe2 mutants is compromised in mature tumors. (A) Tumors collected from infected mature maize plants 14 days postinoculation. The cross of compatible wild-type strains resulted in production of mature tumors in a 14-day period in contrast to the immature tumors produced by the cross of mfe2 mutant strains. (B) Subset of tumors collected from mature maize plants 14 days postinoculation. All mfe2 mutant tumors were white in appearance and contained sporogenic hyphae, indicating that fungal development was not yet complete. (C) The cross section of the tumors shown in panel A. The wild-type “black” tumors were filled with melanized teliospores. The mutant “white” tumors were filled mostly with sporogenic hyphae and immature sexual spores that were not yet melanized and therefore had not completed development. Bar, 10 μm.
DISCUSSION
We have shown in this report that deletion of the mfe2 gene encoding a multifunctional β-oxidation enzyme influences both the morphological response to fatty acids and the growth of U. maydis on these substrates. Furthermore, loss of mfe2 altered lipid accumulation and fatty acid composition within mutant cells. These observations on cells grown in culture established a foundation to examine growth of the mutant in the host, and we observed attenuated virulence in both maize seedlings and developing ears. The simplest explanation is that the defect in virulence results from a metabolic deficiency that prevents proper utilization of host nutrients and therefore delays extensive proliferation in planta. However, it is also possible that specific fatty acids present in the host play a signaling role that is important for successful infection-related development (e.g., filamentation) for U. maydis. A defect in lipid metabolism in the pathogen could interfere with the processing of these putative signals. Additionally, fungal lipid metabolism might influence plant lipid signaling and indirectly interfere with or promote a defense response that alters filamentous proliferation. As discussed below, these results have implications for understanding fungal biotrophy with respect to nutritional requirements, pathogen perception of the host environment, and plant defense.
Fungal phytopathogenesis and lipid utilization.
Evidence from several phytopathogenic fungi indicates that lipid metabolism is critical during the early stages of infection that involve spore germination, production of infection structures (i.e., appressoria formation), and penetration. For example, lipid droplets move to the appressorium following germination of conidia in Magnaporthe grisea, and degradation in the vacuole appears to contribute to the glycerol accumulation required for generating turgor pressure during penetration (74). A similar mobilization and utilization of lipid reserves may occur in Colletotrichum species (5). For the biotrophic fungus Blumeria graminis, microarray data also indicate that genes for lipid catabolism are highly expressed in early stages of infection and decrease in expression in later stages (11). Consistently, storage lipids in conidia of B. graminis are used during penetration and colonization of the host and reaccumulate later in the life cycle when new conidia are formed. Transcriptome analyses also indicate that lipid catabolism is important throughout the germination and penetration stages of infection of B. graminis (75, 76). In a genetic test of the role of peroxisomal function in fungal phytopathogenesis, Kimura et al. (38) showed that loss of peroxisomal function through mutation of the clapex6 gene resulted in a defect in appressorial penetration by C. lagenarium. However, the fungus was still able to proliferate in the host when introduced through wounds, suggesting that lipid catabolism was not required for invasive growth. This pathogen displays biotrophy early in infection with a subsequent switch to nectrotrophic growth that may overcome the requirement for peroxisomal function. Although the importance of β-oxidation in phytopathogenesis has not been studied in detail, accumulating evidence supports the importance of the glyoxylate cycle that produces glucose from the acetyl-CoA that results from the breakdown of fatty acids. Specifically, the glyoxylate cycle is required for full virulence in the phytopathogenic fungi Tapesia yallundae, Leptosphaeria maculans, M. grisea, and Stagonospora nodorum and the human fungal pathogen C. albicans (12, 35, 47, 48, 72, 89). Furthermore, enzymes for lipid degradation including secreted lipases also contribute to virulence in some phytopathogens such as Botrytis cinerea, Alternaria brassicicola, and Fusarium graminearum (9, 17, 88).
In contrast to the situation in other phytopathogens, peroxisomal β-oxidation appears to be less important during the early stages of the disease development in U. maydis. Specifically, the processes of teliospore germination, haploid cell mating to form the infectious cell type, and plant surface penetration were unaffected in the mfe2 mutants. U. maydis also differs from many of the well-studied phytopathogenic fungi in that it does not produce true appressoria (i.e., rounded, melanized structures) but instead produces appressorium-like swellings at the tips of infectious dikaryons to penetrate the plant surface. U. maydis also does not make obvious haustoria to acquire nutrients from the host (69, 70). In contrast, our analysis indicated that loss of mfe2 influenced later stages of disease development including extensive proliferation of branched hyphae and sporulation in planta. U. maydis generally infects actively growing meristematic tissue in maize, resulting in tumors that are often found in the immature, expanding tissue at the base of the leaf (14, 92). Glycolipids and phospholipids would potentially be available to the fungus in this tissue and these generally contain the following fatty acids: C16:0, C18:0, C18:1, C18:2, and C18:3 (28, 45). Traces of C14:0, C16:1, and C20:0 are also found. Fatty acids are synthesized in plastids in developing plant tissue, and the amount of lipid increases in parallel to the plastid development in green developing maize leaves (45, 53). A similar fatty acid composition is also found in maize kernels (33, 61). It is not yet clear whether U. maydis would have access to these lipids during infection and tumor formation. Upon infection, the fungus initially grows intracellularly in epidermal cells, parenchyma cells, and cells of the vascular bundles. Later, the fungus grows mostly intercellularly as highly branched hyphae, some of which protrude into plant cells (70). Banuett and Herskowitz (3) also described the proliferation of hyphae within host cells with the eventual rupture of the cells during sporulation. In general, examination of tumor sections reveal aggregates of fungal hyphae in the process of sporulation surrounded by hypertrophied host cells that appear mainly empty (70). Callow and Ling (14) also reported that plastids and starch disappear during tumor formation leaving empty host cells around areas of abundant sporulation. The lipid composition of the tumor tissue on maize ears has actually been characterized in some detail because these galls are an edible delicacy in Mexico (known as cuitlacoche or “corn truffle”). Based on nutritional analysis, LCFA are abundant components of cuitlacoche, particularly oleic and linoleic acid followed by linolenic and palmitic acid (28, 85, 86). Oleic, linoleic, and palmitic acids are also known to be predominant fatty acid species in teliospores of U. maydis (26). Of course, tumor tissue represents a mixture of plant and fungal material, and it is therefore difficult to separate the relative contributions to fatty acid content.
Possible roles for lipid signaling in fungal morphogenesis and plant defense.
Part of our investigation into the role of mfe2 considered the question of whether a defect in fatty acid metabolism would influence the morphological transition leading to infectious hyphae. This question was motivated by our previous observations that exogenous lipids trigger the dimorphic switch from budding to hyphal growth in culture (40). Notably, we found that mfe2 was required for filamentation in response to some but not all fatty acids. For example, palmitic acid (C16:0) triggered the formation of unbranched hyphae that resembled the mating filaments and the initial infectious dikaryons that usually form on a leaf surface during early stages of infection. Oleic acid and linoleic acid induced highly branched filaments in wild-type cells, but the mfe2 mutants formed filaments with very short branches on oleic acid and did not respond morphologically to linoleic acid. Unlike wild-type strains, growth of the mutants was limited on both fatty acids. These results suggests that exogenous linoleic acid may have a specific signaling role either directly or after processing to form derivatives such as oxylipins.
Precedent for the regulation of fungal morphogenesis by fatty acids and oxylipins comes from studies on sporulation, secondary metabolite production and sexual development in filamentous fungi (25, 41, 79-84). For example, the influence of oxylipins has been studied in detail in Aspergillus nidulans, and it has been shown that hydroxylated oleic, linoleic, and linolenic acids constitute an endogenous mixture of oxylipin hormones (psi factors) that control the timing and balance of meiotic and mitotic spore development (15, 16, 79, 81-84). More recent work revealed that mutants defective in the fatty acid oxygenases for psi factor production (ppo genes) had reduced production of the mycotoxin sterigmatocystin, reduced peanut seed colonization, and increased penicillin production (80). With regard to potential oxylipin metabolism, a polypeptide (Ssp1) with similarity to linoleate diol synthase has been found to be abundant in U. maydis teliospores and a function in the mobilization of storage lipids has been proposed (34). However, deletion of the ssp1 gene does not result in an obvious phenotype. It is possible that U. maydis responds to derivatives of fatty acids and that mfe2 and β-oxidation may be required to produce specific intermediates that are further modified to produce signaling lipid/fatty acid molecules. It may also be the case that the loss of mfe2 could have an indirect effect on morphogenesis by influencing cellular fatty acid composition as demonstrated by our chemical analyses. Clearly, further investigation of the specificity of signaling among fatty acids and derivatives such as oxylipins is needed.
The mfe2 mutation impaired symptom development during infection but did not completely abolish fungal growth within the plant. In general, biotrophic fungi like U. maydis evade plant defense mechanisms by camouflage or by suppression or actively defend themselves by mechanisms such as detoxification of host metabolites (65). It is possible that an enhanced defense response occurs upon infection by mfe2 mutants because of the altered chemical environment caused by the metabolic defect in the pathogen. That is, a change in lipid composition or signaling could trigger a defense response through activation of jasmonic acid signaling and the defense responses of the salicylic acid-dependent pathway. Lipids clearly have an impact on the plant defense response through a variety of mechanisms (66). For example, fatty acids play an important role in modulating signaling between the salicylic acid- and jasmonic acid-dependent defense pathways, and recent studies have uncovered an important role for lipids also in the activation of systemic acquired resistance (36, 51, 55). In general, little is known about the defense response of maize to infection by U. maydis. Basse (7) recently described transcriptional changes for maize genes related to metabolism and development in response to U. maydis infection as well as putative defense responses of the host. Evidence was obtained to suggest that U. maydis is capable of suppressing a defense response based on the observation that a weakly proliferative mutant triggered expression of the pathogenesis related gene PR-1. These observations suggest that it may be informative to examine defense-related gene expression during infection with mfe2 mutants, although we favor the hypothesis that a nutritional defect results in reduced growth in planta.
In summary, we have demonstrated the importance of a β-oxidation function for growth on fatty acids in culture and for biotrophic infection. However, many additional questions remain about the importance of lipid metabolism in U. maydis during infection including the potential role of lipases, the extent of lipid utilization, and the specificity of potential signals from fatty acids and their derivatives. In addition, the genome sequence suggests that the fungus may also have a mitochondrial β-oxidation system, and the contribution of this system to infection remains to be explored.
Acknowledgments
We thank C. I. Keeling for technical assistance with GC/MS analysis and K. H. Rensing for help with the electron microscopy. We thank A. L. Samuels and V. Katavic for helpful discussions and technical advice and L. Kunst and X. Li for critical reading of the manuscript.
This work was funded by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (to J.W.K.) and the UBC University Graduate Fellowship (to J.K.).
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, D. L., J. D. Egan, M. E. Mayorga, and S. E. Gold. 2000. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol. Plant-Microbe Interact. 13:781-786. [DOI] [PubMed] [Google Scholar]
- 3.Banuett, F., and I. Herskowitz. 1996. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122:2965-2976. [DOI] [PubMed] [Google Scholar]
- 4.Banuett, F., and I. Herskowitz. 1994. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 8:1367-1378. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa, A. C., A. E. Carmo, L. Graf, R. Tomaz, C. F. Souza, J. Mendes, M. A. Randi, D. Buchi, and R. J. Schadeck. 2006. Morphology and lipid body and vacuole dynamics during secondary conidia formation in Colletotrichum acutatum: laser scanning confocal analysis. Can. J. Microbiol. 52:117-124. [DOI] [PubMed] [Google Scholar]
- 6.Barrett, K. J., S. E. Gold, and J. W. Kronstad. 1993. Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol. Plant-Microbe Interact. 6:274-283. [DOI] [PubMed] [Google Scholar]
- 7.Basse, C. W. 2005. Dissecting defense-related and developmental transcriptional responses of maize during Ustilago maydis infection and subsequent tumor formation. Plant Physiol. 138:1774-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berto, P., P. Commenil, L. Belingheri, and B. Dehorter. 1999. Occurrence of a lipase in spores of Alternaria brassicicola with a crucial role in the infection of cauliflower leaves. FEMS Microbiol. Lett. 180:183-189. [DOI] [PubMed] [Google Scholar]
- 10.Boothroyd, B., J. A. Thorn, and R. H. Haskins. 1956. Biochemistry of the ustilaginales. XII. Characterization of extracellular glycolipids produced by Ustilago sp. Can. J. Biochem. Physiol. 34:10-14. [PubMed] [Google Scholar]
- 11.Both, M., M. Csukai, M. P. Stumpf, and P. D. Spanu. 2005. Gene expression profiles of Blumeria graminis indicate dynamic changes to primary metabolism during development of an obligate biotrophic pathogen. Plant Cell 17:2107-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowyer, P., E. Mueller, and J. Lucas. 2000. Use of an isocitrate lyase promoter-GFP fusion to monitor carbon metabolism of the plant pathogen Tapesia yallundae during infection of wheat. Mol. Plant Pathol. 1:253-262. [DOI] [PubMed] [Google Scholar]
- 13.Brachmann, A., G. Weinzierl, J. Kamper, and R. Kahmann. 2001. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42:1047-1063. [DOI] [PubMed] [Google Scholar]
- 14.Callow, J. A., and I. T. Ling. 1973. Histology of neoplasms and chlorotic lesions in maize seedlings following the injection of sporidia of Ustilago maydis (DC) Corda. Physiol. Plant Pathol. 3:489-494. [Google Scholar]
- 15.Calvo, A. M., H. W. Gardner, and N. P. Keller. 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276:25766-25774. [DOI] [PubMed] [Google Scholar]
- 16.Champe, S. P., P. Rao, and A. Chang. 1987. An endogenous inducer of sexual development in Aspergillus nidulans. J. Gen. Microbiol. 133:1383-1387. [DOI] [PubMed] [Google Scholar]
- 17.Commenil, P., L. Belingheri, M. Sancholle, and B. Dehorter. 1995. Purification and properties of an extracellular lipase from the fungus Botrytis cinerea. Lipids 30:351-356. [DOI] [PubMed] [Google Scholar]
- 18.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berriosqq, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 19.de Hoop, M. J., and G. Ab. 1992. Import of proteins into peroxisomes and other microbodies. Biochem. J. 286:657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durrenberger, F., R. D. Laidlaw, and J. W. Kronstad. 2001. The hgl1 gene is required for dimorphism and teliospore formation in the fungal pathogen Ustilago maydis. Mol. Microbiol. 41:337-348. [DOI] [PubMed] [Google Scholar]
- 21.Durrenberger, F., K. Wong, and J. W. Kronstad. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 95:5684-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fossa, A., A. Beyer, E. Pfitzner, B. Wenzel, and W. H. Kunau. 1995. Molecular cloning, sequencing and sequence analysis of the fox-2 gene of Neurospora crassa encoding the multifunctional beta-oxidation protein. Mol. Gen. Genet. 247:95-104. [DOI] [PubMed] [Google Scholar]
- 23.Gold, S., G. Duncan, K. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 24.Gold, S. E., S. M. Brogdon, M. E. Mayorga, and J. W. Kronstad. 1997. The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 9:1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodrich-Tanrikulu, M., K. Howe, A. Stafford, and M. A. Nelson. 1998. Changes in fatty acid composition of Neurospora crassa accompany sexual development and ascospore germination. Microbiology 144:1713-1720. [DOI] [PubMed] [Google Scholar]
- 26.Gunasekaran, M., J. L. Bushnell, and D. J. Weber. 1972. Comparative studies on lipid components of Ustilago bullata and Ustilago maydis spores. Res. Commun. Chem. Pathol Pharmacol. 3:621-628. [PubMed] [Google Scholar]
- 27.Hartmann, H. A., R. Kahmann, and M. Bolker. 1996. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 15:1632-1641. [PMC free article] [PubMed] [Google Scholar]
- 28.Hawke, J. C., M. G. Rumsby, and R. M. Leech. 1974. Lipid biosynthesis in green leaves of developing maize. Plant Physiol. 53:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewald, S., K. Josephs, and M. Bolker. 2005. Genetic analysis of biosurfactant production in Ustilago maydis. Appl. Environ Microbiol. 71:3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiltunen, J. K., B. Wenzel, A. Beyer, R. Erdmann, A. Fossa, and W. H. Kunau. 1992. Peroxisomal multifunctional beta-oxidation protein of Saccharomyces cerevisiae. Molecular analysis of the fox2 gene and gene product. J. Biol. Chem. 267:6646-6653. [PubMed] [Google Scholar]
- 31.Holliday, R. 1961. The genetics of Ustilago maydis. Genet. Res. Camb. Soc. 2:204-230. [Google Scholar]
- 32.Holliday, R. 1974. Ustilago maydis, p. 575-595. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 33.Hsing, Y. C., J. M. Widholm, and R. W. Rinne. 1993. Lipid metabolism in maize tissue-culture. J. Plant Physiol. 142:360-365. [Google Scholar]
- 34.Huber, S. M., F. Lottspeich, and J. Kamper. 2002. A gene that encodes a product with similarity to dioxygenases is highly expressed in teliospores of Ustilago maydis. Mol. Genet. Genomics 267:757-771. [DOI] [PubMed] [Google Scholar]
- 35.Idnurm, A., and B. J. Howlett. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot. Cell 1:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kachroo, A., L. Lapchyk, H. Fukushige, D. Hildebrand, D. Klessig, and P. Kachroo. 2003. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 15:2952-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamiryo, T., Y. Sakasegawa, and H. Tan. 1989. Expression and transport of Candida tropicalis peroxisomal acyl-coenzyme A oxidase in the yeast Candida maltosa. Agric. Biol Chem. 53:179-186. [Google Scholar]
- 38.Kimura, A., Y. Takano, I. Furusawa, and T. Okuno. 2001. Peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium. Plant Cell 13:1945-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura, K., M. Yamaoka, and Y. Kamisaka. 2004. Rapid estimation of lipids in oleaginous fungi and yeasts using Nile red fluorescence. J. Microbiol. Methods 56:331-338. [DOI] [PubMed] [Google Scholar]
- 40.Klose, J., M. M. Moniz de Sa, and J. W. Kronstad. 2004. Lipid-induced filamentous growth in Ustilago maydis. Mol. Microbiol. 52:823-835. [DOI] [PubMed] [Google Scholar]
- 41.Kock, J. L., C. J. Strauss, C. H. Pohl, and S. Nigam. 2003. The distribution of 3-hydroxy oxylipins in fungi. Prostaglandins Other Lipid Mediat. 71:85-96. [DOI] [PubMed] [Google Scholar]
- 42.Kunau, W. H., V. Dommes, and H. Schulz. 1995. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34:267-342. [DOI] [PubMed] [Google Scholar]
- 43.Kunau, W. H., and A. Hartig. 1992. Peroxisome biogenesis in Saccharomyces cerevisiae. Antonie Leeuwenhoek 62:63-78. [DOI] [PubMed] [Google Scholar]
- 44.Lee, N., and J. W. Kronstad. 2002. ras2 controls morphogenesis, pheromone response, and pathogenicity in the fungal pathogen Ustilago maydis. Eukaryot. Cell 1:954-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leech, R. M., M. G. Rumsby, and W. W. Thomson. 1973. Plastid differentiation, acyl lipid, and fatty acid changes in developing green maize leaves. Plant Physiol. 53:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemieux, R. U., J. A. Thorn, C. Brice, and R. H. Haskins. 1951. Biochemistry of the ustilaginales. II. Isolation and partial characterization of ustilagic acid. Can. J. Chem. 29:409-414. [DOI] [PubMed] [Google Scholar]
- 47.Lorenz, M. C., J. A. Bender, and G. R. Fink. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 49.Luo, Y., I. V. Karpichev, R. A. Kohanski, and G. M. Small. 1996. Purification, identification, and properties of a Saccharomyces cerevisiae oleate-activated upstream activating sequence-binding protein that is involved in the activation of POX1. J. Biol. Chem. 271:12068-12075. [DOI] [PubMed] [Google Scholar]
- 50.Maggio-Hall, L. A., and N. P. Keller. 2004. Mitochondrial beta-oxidation in Aspergillus nidulans. Mol. Microbiol. 54:1173-1185. [DOI] [PubMed] [Google Scholar]
- 51.Maldonado, A. M., P. Doerner, R. A. Dixon, C. J. Lamb, and R. K. Cameron. 2002. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419:399-403. [DOI] [PubMed] [Google Scholar]
- 52.Mayorga, M. E., and S. E. Gold. 1999. A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34:485-497. [DOI] [PubMed] [Google Scholar]
- 53.Moore, T. S., and G. D. Troyer. 1983. Phospholipids and metabolism. In W. W. Thomson, B. J. Mudd, and M. Gibbs (ed.), Biosynthesis and function of plant lipids. American Society of Plant Physiologists, Rockville, Md.
- 54.Moreno de la Garza, M., U. Schultz-Borchard, J. W. Crabb, and W. H. Kunau. 1985. Peroxisomal beta-oxidation system of Candida tropicalis. Purification of a multifunctional protein possessing enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase and 3-hydroxyacyl-CoA epimerase activities. Eur. J. Biochem. 148:285-291. [DOI] [PubMed] [Google Scholar]
- 55.Nandi, A., R. Welti, and J. Shah. 2004. The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell 16:465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novikov, D. K., G. F. Vanhove, H. Carchon, S. Asselberghs, H. J. Eyssen, P. P. Van Veldhoven, and G. P. Mannaerts. 1994. Peroxisomal beta-oxidation. Purification of four novel 3-hydroxyacyl-CoA dehydrogenases from rat liver peroxisomes. J. Biol. Chem. 269:27125-27135. [PubMed] [Google Scholar]
- 57.Pataky, J. K. 1991. Production of cuitlacoche (Ustilago maydis (DS) Corda) on sweet corn. HortScience. 26:1374-1377. [Google Scholar]
- 58.Pataky, J. K. 1990. Production of huitlacoche, Ustilago maydis. Phytopathology 80:1044. [Google Scholar]
- 59.Purdue, P. E., and P. B. Lazarow. 1994. Peroxisomal biogenesis: multiple pathways of protein import. J. Biol. Chem. 269:30065-30068. [PubMed] [Google Scholar]
- 60.Qin, Y. M., M. S. Marttila, A. M. Haapalainen, K. M. Siivari, T. Glumoff, and J. K. Hiltunen. 1999. Yeast peroxisomal multifunctional enzyme: (3R)-hydroxyacyl-CoA dehydrogenase domains A and B are required for optimal growth on oleic acid. J. Biol. Chem. 274:28619-28625. [DOI] [PubMed] [Google Scholar]
- 61.Ratcliff, S. L., D. O. J. Wilson, E. A. Knott, and S. K. Mohan. 1993. Free fatty acids in shrunken-2 sweet corn seed. Crop Sci. 33:871-873. [Google Scholar]
- 62.Requena, N., P. Fuller, and P. Franken. 1999. Molecular characterization of GmFOX2, an evolutionarily highly conserved gene from the mycorrhizal fungus Glomus mosseae, down-regulated during interaction with rhizobacteria. Mol. Plant-Microbe Interact. 12:934-942. [DOI] [PubMed] [Google Scholar]
- 63.Sambrook, J. E., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 64.Schmitt, M. E., T. A. Brown, and B. L. Trumpower. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18:3091-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulze-Lefert, P., and R. Panstruga. 2003. Establishment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu. Rev. Phytopathol. 41:641-667. [DOI] [PubMed] [Google Scholar]
- 66.Shah, J. 2005. Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu. Rev. Phytopathol. 43:229-260. [DOI] [PubMed] [Google Scholar]
- 67.Small, G. M., L. J. Szabo, and P. B. Lazarow. 1988. Acyl-CoA oxidase contains two targeting sequences each of which can mediate protein import into peroxisomes. EMBO J. 7:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, J. J., T. W. Brown, G. A. Eitzen, and R. A. Rachubinski. 2000. Regulation of peroxisome size and number by fatty acid beta-oxidation in the yeast Yarrowia lipolytica. J. Biol. Chem. 275:20168-20178. [DOI] [PubMed] [Google Scholar]
- 69.Snetselaar, K. M., and C. W. Mims. 1993. Infection of maize stigmas by Ustilago maydis: light and electron microscopy. Phytopathology 83:843-850. [Google Scholar]
- 70.Snetselaar, K. M., and C. W. Mims. 1994. Light and electron microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 98:347-355. [Google Scholar]
- 71.Snetselaar, K. M., and C. W. Mims. 1992. Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia 84:193-203. [Google Scholar]
- 72.Solomon, P. S., R. C. Lee, T. J. Wilson, and R. P. Oliver. 2004. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol. Microbiol. 53:1065-1073. [DOI] [PubMed] [Google Scholar]
- 73.Stanway, C. A., J. M. Gibbs, and E. Berardi. 1995. Expression of the FOX1 gene of Saccharomyces cerevisiae is regulated by carbon source, but not by the known glucose repression genes. Curr. Genet. 27:404-408. [DOI] [PubMed] [Google Scholar]
- 74.Thines, E., R. W. Weber, and N. J. Talbot. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas, S. W., M. A. Glaring, S. W. Rasmussen, J. T. Kinane, and R. P. Oliver. 2002. Transcript profiling in the barley mildew pathogen Blumeria graminis by serial analysis of gene expression (SAGE). Mol. Plant-Microbe Interact. 15:847-856. [DOI] [PubMed] [Google Scholar]
- 76.Thomas, S. W., S. W. Rasmussen, M. A. Glaring, J. A. Rouster, S. K. Christiansen, and R. P. Oliver. 2001. Gene identification in the obligate fungal pathogen Blumeria graminis by expressed sequence tag analysis. Fungal Genet. Biol. 33:195-211. [DOI] [PubMed] [Google Scholar]
- 77.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsitsigiannis, D. I., J. W. Bok, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 73:4548-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsitsigiannis, D. I., and N. P. Keller. 2006. Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol. Microbiol. 59:882-892. [DOI] [PubMed] [Google Scholar]
- 81.Tsitsigiannis, D. I., T. M. Kowieski, R. Zarnowski, and N. P. Keller. 2004. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell. 3:1398-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsitsigiannis, D. I., T. M. Kowieski, R. Zarnowski, and N. P. Keller. 2005. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology 151:1809-1821. [DOI] [PubMed] [Google Scholar]
- 83.Tsitsigiannis, D. I., S. Kunze, D. K. Willis, I. Feussner, and N. P. Keller. 2005. Aspergillus infection inhibits the expression of peanut 13S-HPODE-forming seed lipoxygenases. Mol. Plant-Microbe Interact. 18:1081-1089. [DOI] [PubMed] [Google Scholar]
- 84.Tsitsigiannis, D. I., R. Zarnowski, and N. P. Keller. 2004. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 279:11344-11353. [DOI] [PubMed] [Google Scholar]
- 85.Valverde, M. E., and O. Paredes-Lopez. 1993. Production and evaluation of some food properties of huitlacoche (Ustilago maydis). Food Biotechnol. (New York) 7:207-219. [Google Scholar]
- 86.Vanegas, P. E., M. E. Valverde, O. Paredes-Lopez, and J. K. Pataky. 1995. Production of the edible fungus huitlacoche (Ustilago maydis): Effect of maize genotype on chemical composition. J. Ferment. Bioeng. 80:104-106. [Google Scholar]
- 87.Veenhuis, M., M. Mateblowski, W. H. Kunau, and W. Harder. 1987. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast 3:77-84. [DOI] [PubMed] [Google Scholar]
- 88.Voigt, C. A., W. Schafer, and S. Salomon. 2005. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 42:364-375. [DOI] [PubMed] [Google Scholar]
- 89.Wang, Z. Y., C. R. Thornton, M. J. Kershaw, L. Debao, and N. J. Talbot. 2003. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 47:1601-1612. [DOI] [PubMed] [Google Scholar]
- 90.Watson, S. A. 2003. Description, development, structure and composition of the corn kernel, p. 69-101. In P. J. White and L. A. Johnson (ed.), Corn: chemistry and technology, 2nd ed. American Association of Cereal Chemists, St. Paul, Minn.
- 91.Watson, S. A. 1987. Structure and composition. In S. A. Watson, and P. E. Ramsted (ed.), Corn chemistry and technology. American Associations of Cereal Chemists, St. Paul, Minn.
- 92.Wenzler, H., and F. Meins. 1987. Persistent changes in the proliferative capacity of maize leaf tissues induced by Ustilago infection. Physiol. Mol. Plant Pathol. 30:309-319. [Google Scholar]