Abstract
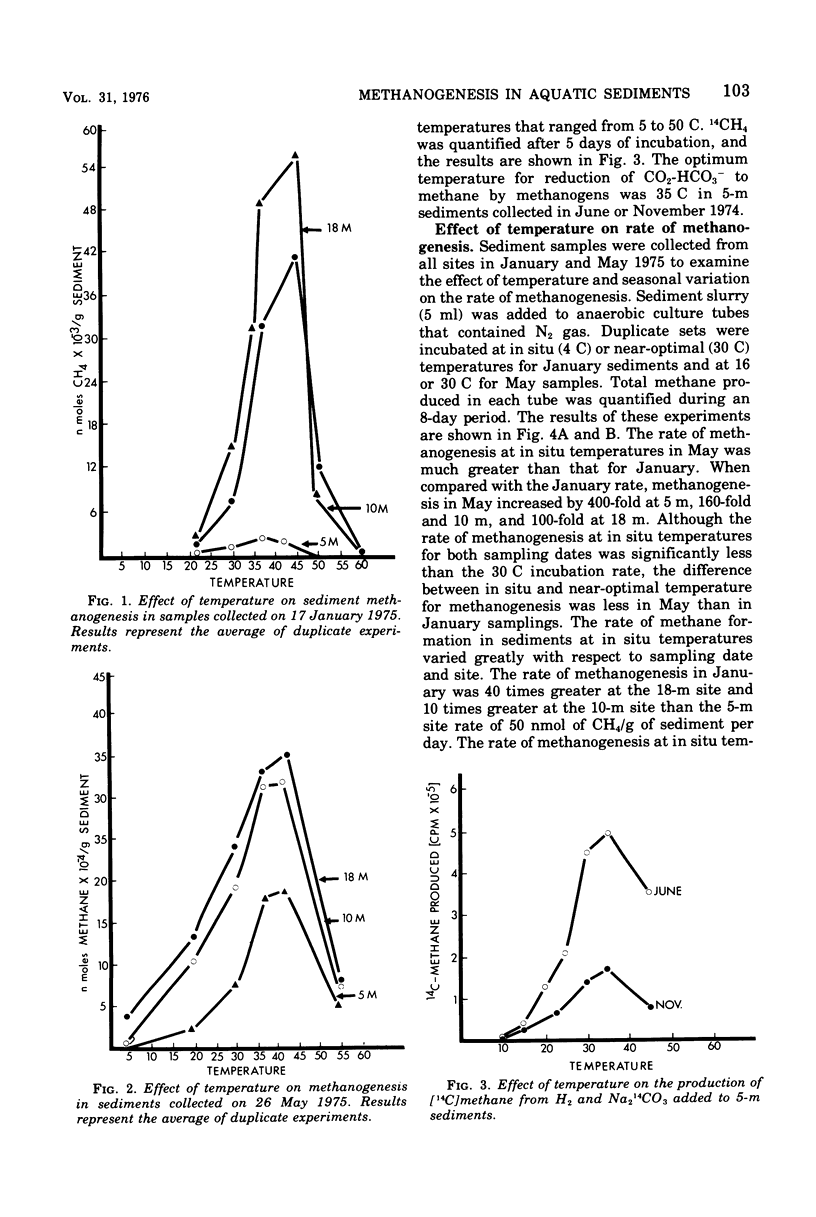
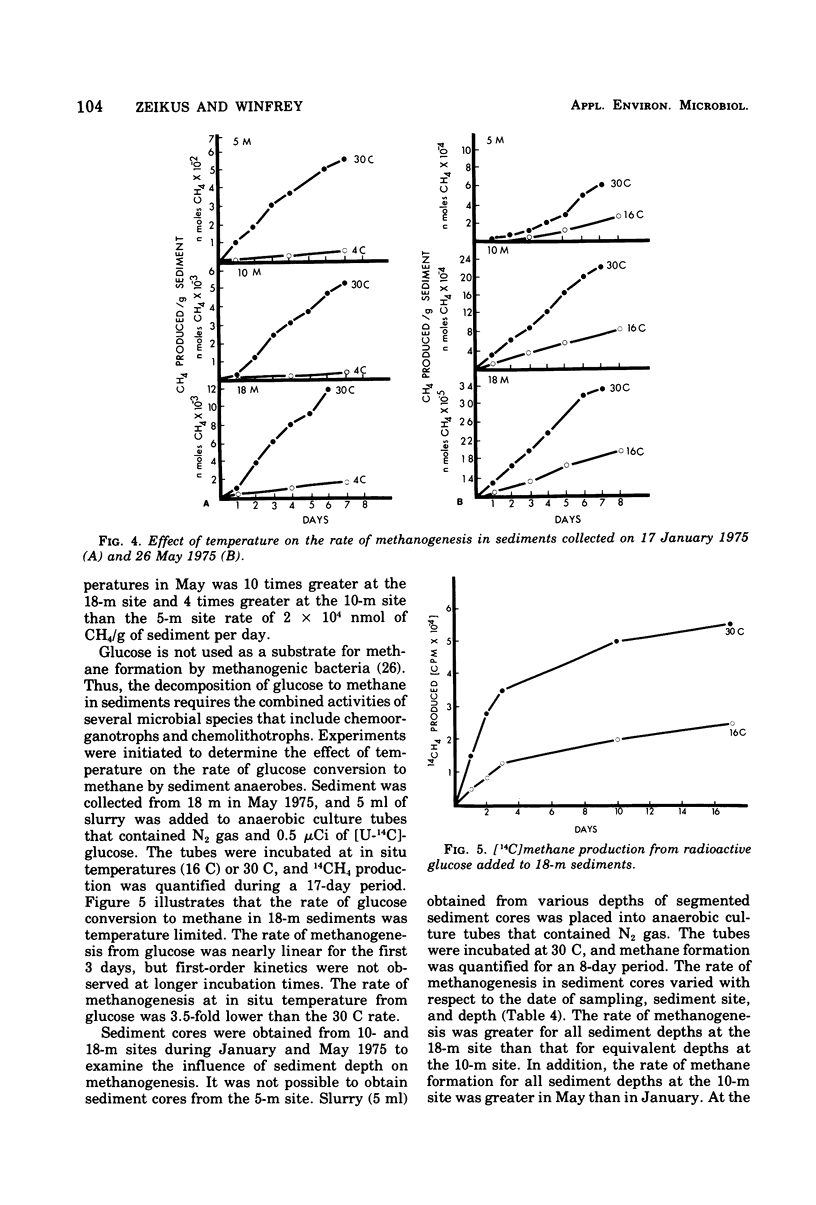
Microbial methanogenesis was examined in sediments collected from Lake Mendota, Wisconsin, at water depths of 5, 10, and 18 m. The rate of sediment methanogenesis was shown to vary with respect to sediment site and depth, sampling date, in situ temperature, and number of methanogens. Increased numbers of methanogenic bacteria and rates of methanogenesis correlated with increased sediment temperature during seasonal change. The greatest methanogenic activity was observed for 18-m sediments throughout the sampling year. As compared with shallower sediments, 18-m sediment was removed from oxygenation effects and contained higher amounts of ammonia, carbonate, and methanogenic bacteria, and the population density of methanogens fluctuated less during seasonal change. Rates of methanogenesis in 18-m sediment cores decreased with increasing sediment depth. The optimum temperature, 35 to 42 C, for sediment methanogenesis was considerably higher than the maximum observed in situ temperature of 23 C. The conversion of H2 and [14C]carbonate to [14C]methane displayed the same temperature optimum when these substrates were added to sediments. The predominant methanogenic population had simple nutritional requirements and were metabolically active at 4 to 45 C. Hydrogen oxidizers were the major nutritional type of sediment methanogens; formate and methanol fermentors were present, but acetate fermentors were not observed. Methanobacterium species were most abundant in sediments although Methanosarcina, Methanococcus, and Methanospirillum species were observed in enrichment cultures. A chemolithotropic species of Methanosarcina and Methanobacterium was isolated in pure culture that displayed temperature optima above 30 C and had simple nutritional requirements.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Deuser W. G., Degens E. T., Harvey G. R., Rubin M. Methane in lake kivu: new data bearing on its origin. Science. 1973 Jul 6;181(4094):51–54. doi: 10.1126/science.181.4094.51. [DOI] [PubMed] [Google Scholar]
- Keeney D. R., Konrad J. G., Chesters G. Nitrogen distribution in some Wisconsin lake sediments. J Water Pollut Control Fed. 1970 Mar;42(3):411–417. [PubMed] [Google Scholar]
- Martens C. S., Berner R. A. Methane production in the interstitial waters of sulfate-depleted marine sediments. Science. 1974 Sep 27;185(4157):1167–1169. doi: 10.1126/science.185.4157.1167. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Ward J. C. Methane formation in living trees: a microbial origin. Science. 1974 Jun 14;184(4142):1181–1183. doi: 10.1126/science.184.4142.1181. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


