Abstract
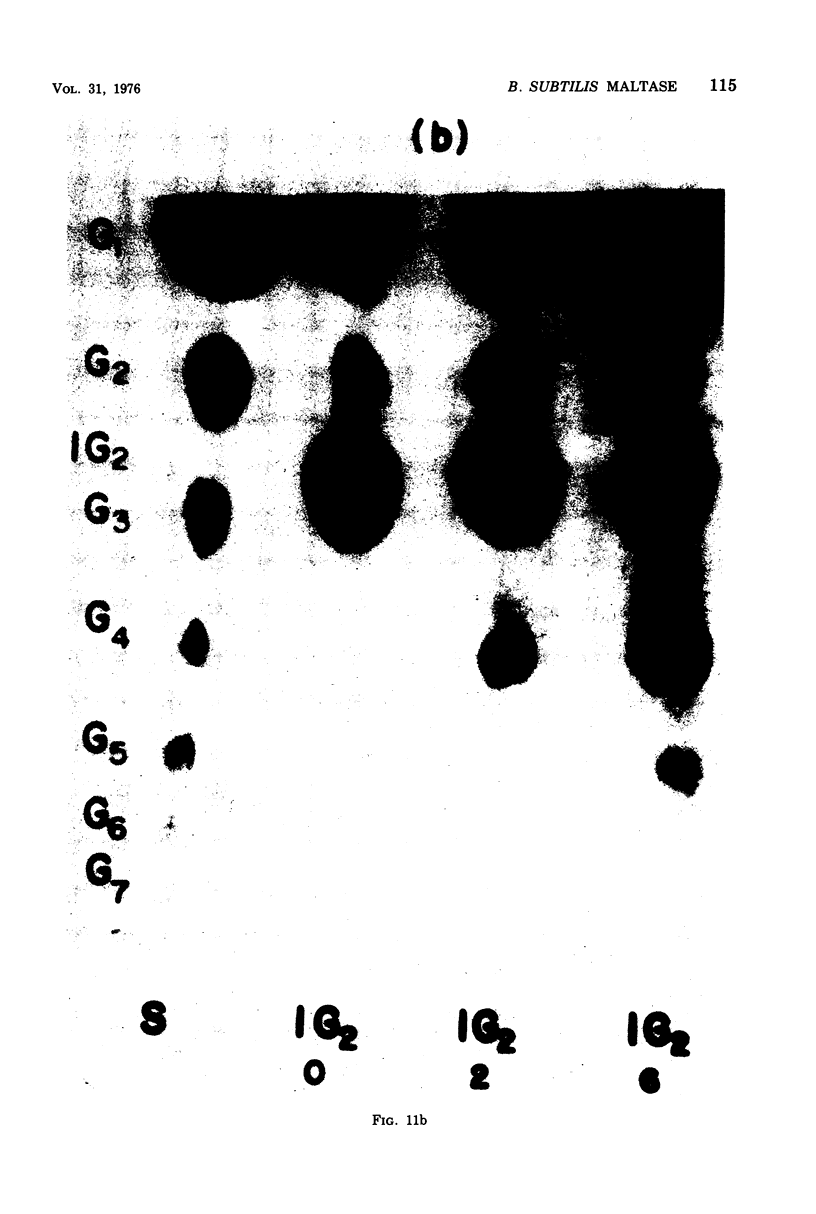
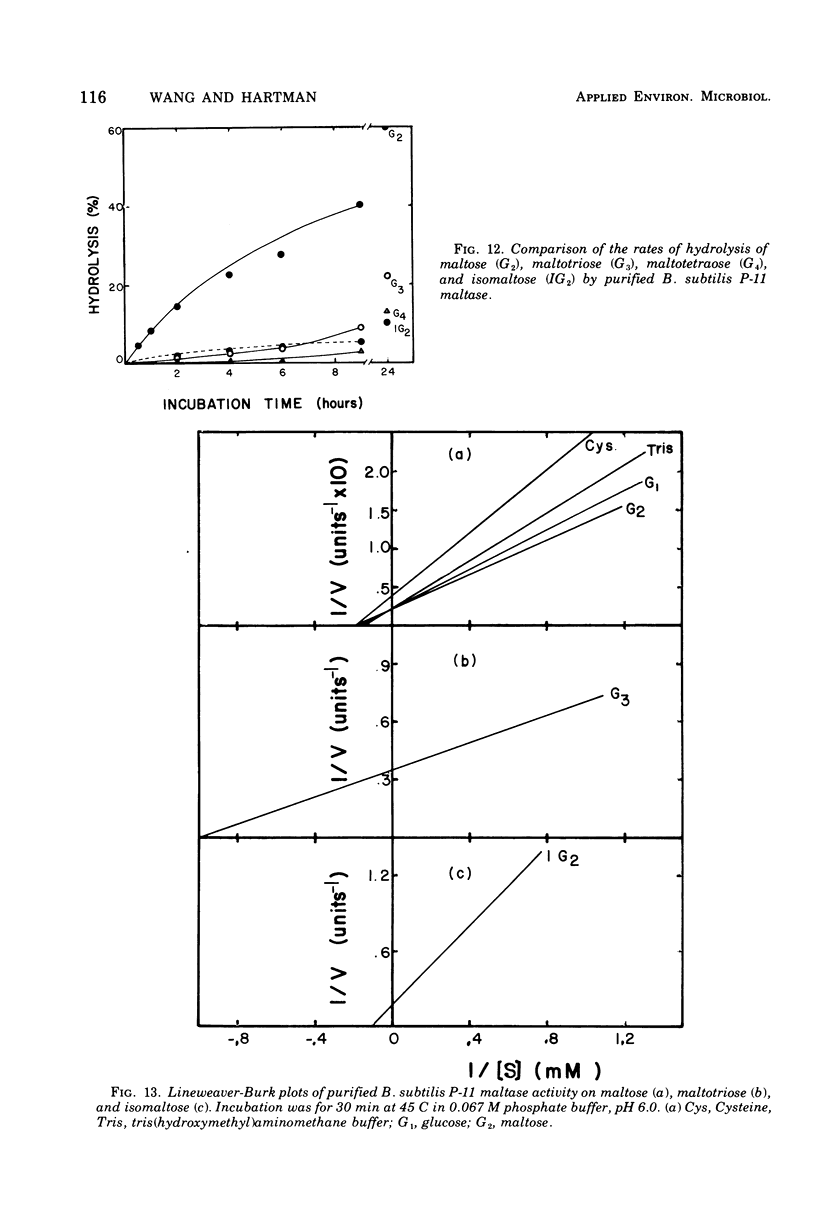
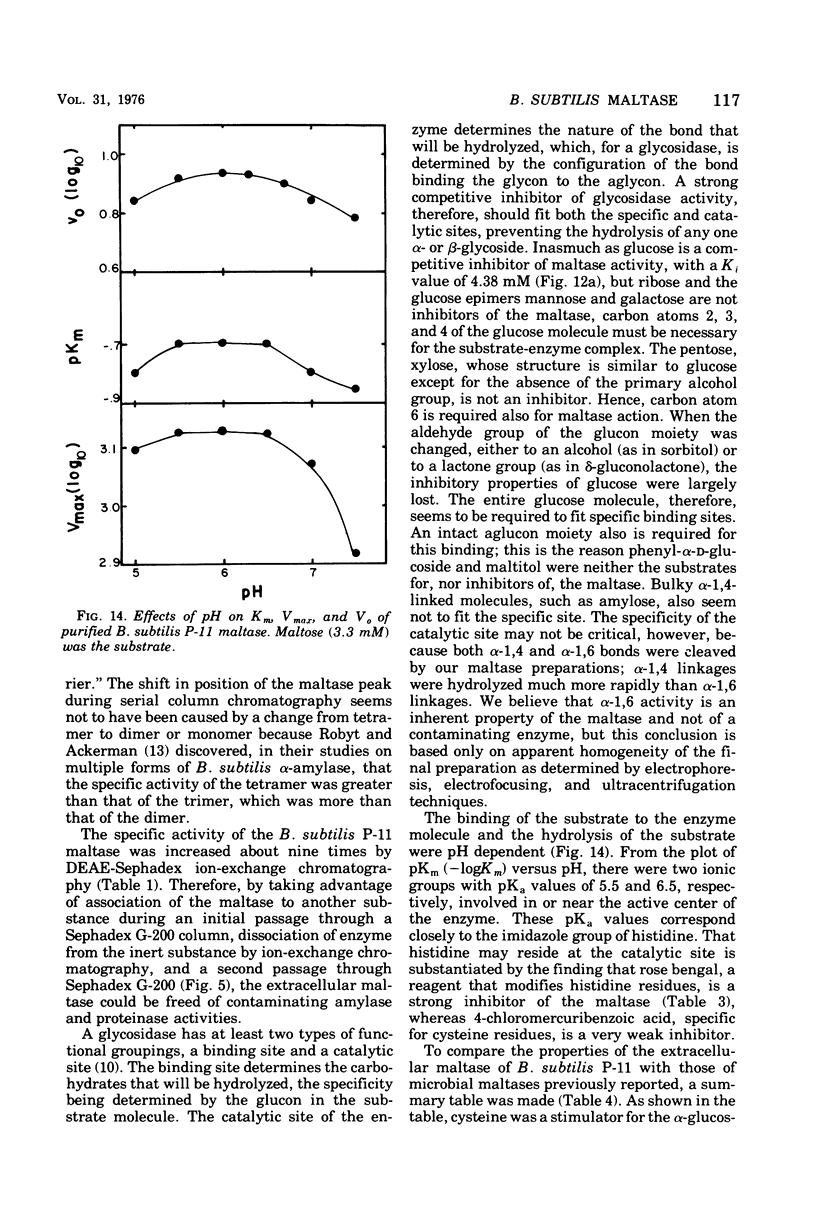
Bacillus subtilis P-11, capable of producing extracellular maltase, was isolated from soil. Maximum enzyme production was obtained on a medium containing 2.0% methyl-alpha-D-glucose, 0.5% phytone, and 0.2% yeast extract. After the removal of cells, extracellular maltase was precipitated by ammonium sulfate (85% saturation). The enzyme was purified by using the following procedures: Sephadex G-200 column chromatography, diethylaminoethyl-Sephadex A-50 ion-exchange column chromatography, and a second Sephadex G-200 column chromatography. A highly purified maltase without amylase or proteinase activities was obtained. Some properties of the extracellular maltase were determined: optimum pH, 6.0; optimum temperature, 45 C, when the incubation time was 30 min; pH stability, within 5.5 to 6.5; heat stability, stable up to 45 C; isoelectric point, pH 6.0 (by gel-isoelectric focusing); molecular weight, 33,000 (by gel filtration with Sephadex G-200); substrate specificity: the relative rates of hydrolysis of maltose, maltotriose, isomaltose, and maltotetraose were 100:15:14:4, respectively, and there was no activity toward alkyl or aryl-alpha-D-glucosides, amylose, or other higher polymers. Transglucosylase activity was present. Glucose and tris(hydroxymethyl)aminomethane were competitive inhibitors with Ki values of 4.54 and 75.08 mM, respectively; cysteine was a noncompetitive inhibitor. Michaelis constants were 5 mM for maltose, 1 mM for maltoriose, and 10 mM for isomaltose. A plot of pKm (-log Km) versus pH revealed two deflection points, one each at 5.5 and 6.5; these probably corresponded to an imidazole group of a histidine residue in or near the active center; this assumption was supported by the strong inhibition of enzyme activity by rose bengal.
Full text
PDF
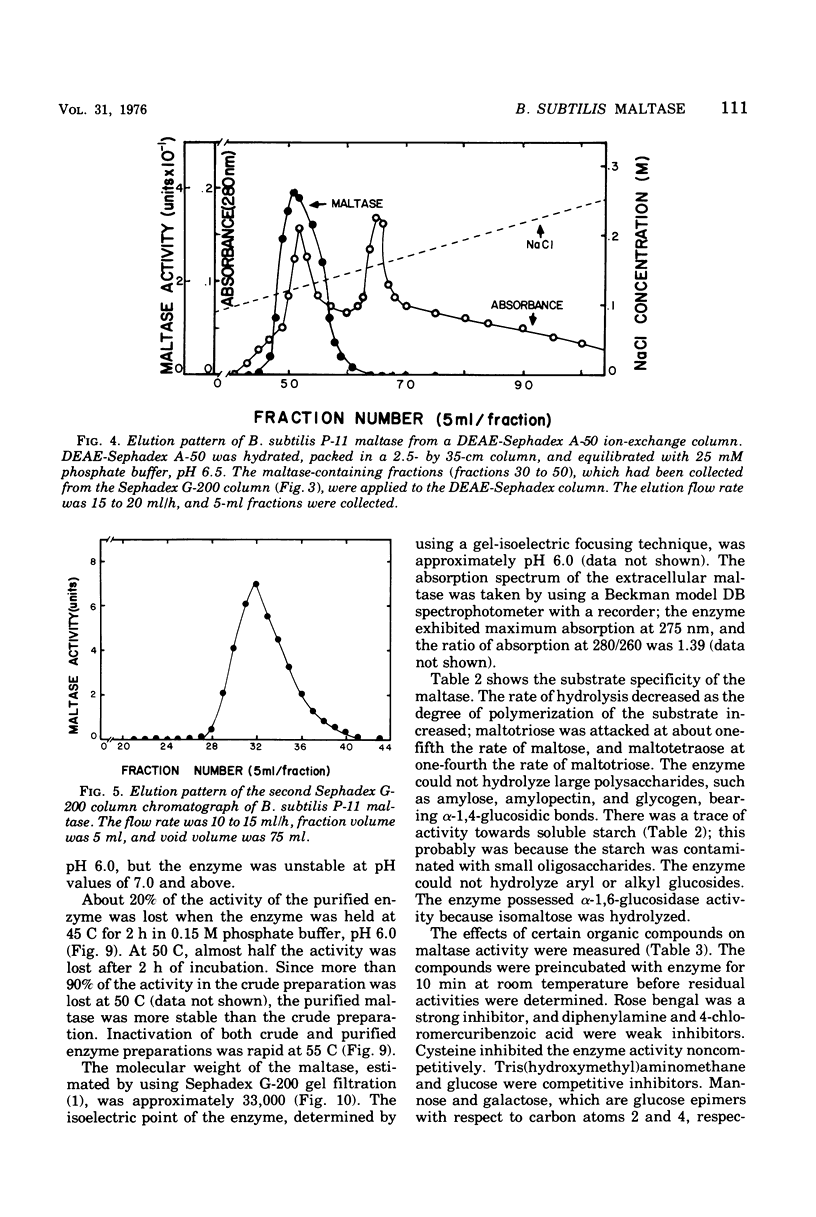
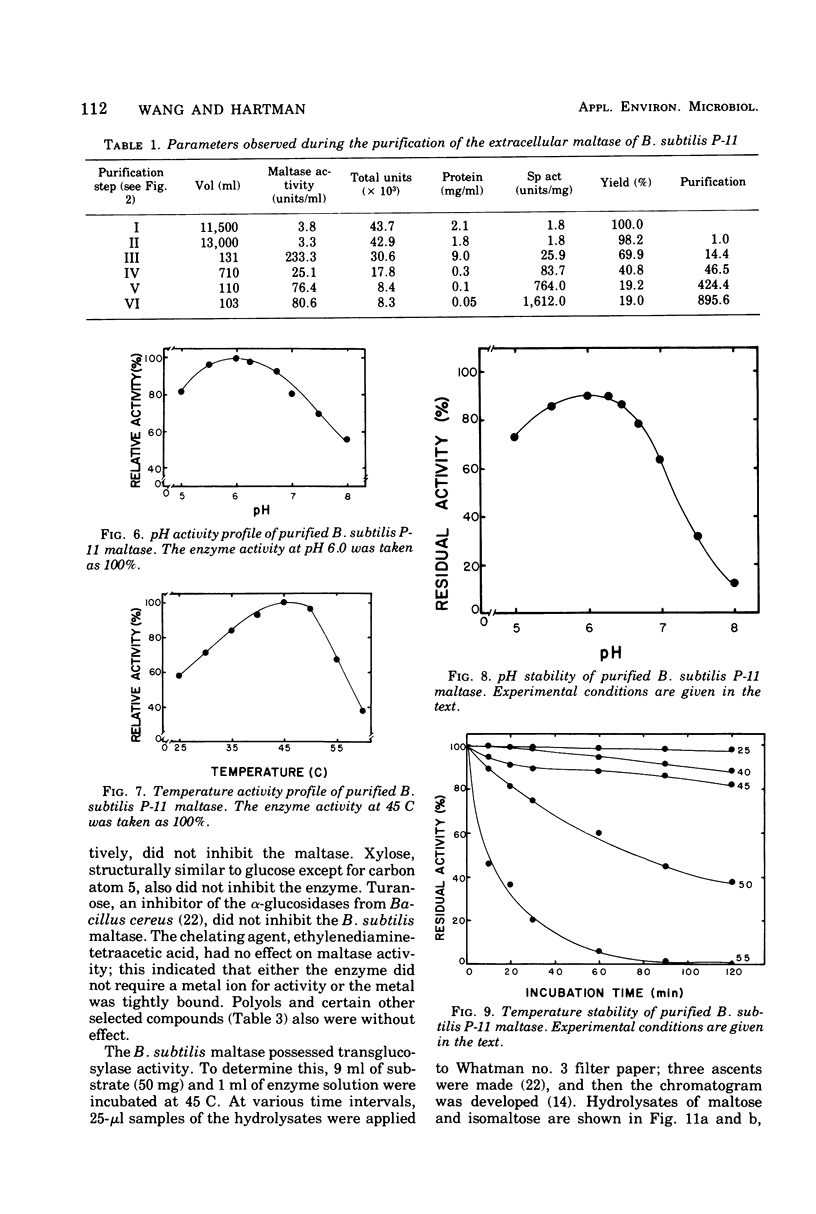
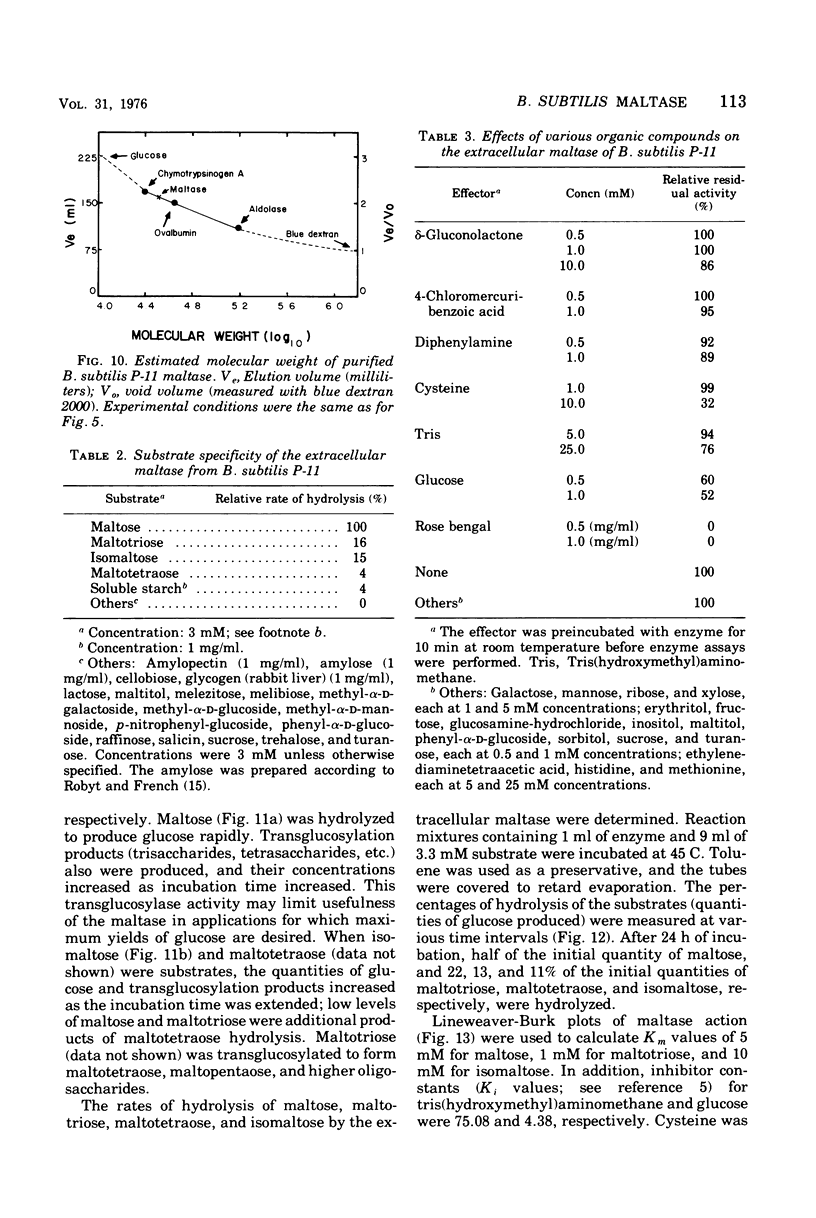
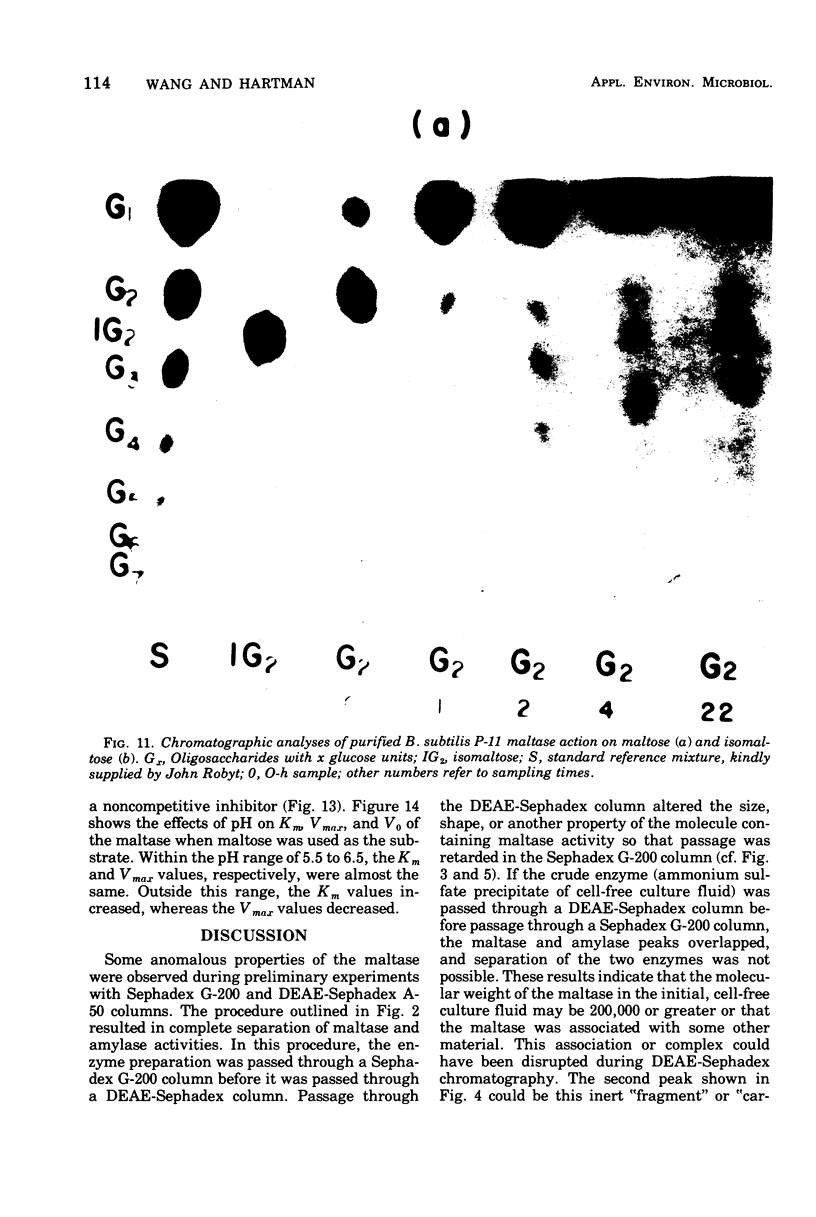

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D., KNAPP D. W. The maltase of Clostridium acetobutylicum; its specificity range and mode of action. J Biol Chem. 1950 Dec;187(2):463–471. [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- Hockenhull D. J., Herbert D. The amylase and maltase of Clostridium acetobutylicum. Biochem J. 1945;39(1):102–106. doi: 10.1042/bj0390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J. Structure and function of amylases. II. Multiple forms of bacillus subtilis -amylase. Arch Biochem Biophys. 1973 Apr;155(2):445–451. doi: 10.1016/0003-9861(73)90135-5. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., French D. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch Biochem Biophys. 1967 Oct;122(1):8–16. doi: 10.1016/0003-9861(67)90118-x. [DOI] [PubMed] [Google Scholar]
- WHELAN W. J., NASR H. The amylase of Clostridium butyricum. Biochem J. 1951 Apr;48(4):416–422. doi: 10.1042/bj0480416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. I. Purification and physical chemical properties of the enzyme amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:417–426. doi: 10.1016/0006-3002(60)90194-3. [DOI] [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. II. General properties and mechanism of action of amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:427–439. doi: 10.1016/0006-3002(60)90195-5. [DOI] [PubMed] [Google Scholar]