Abstract
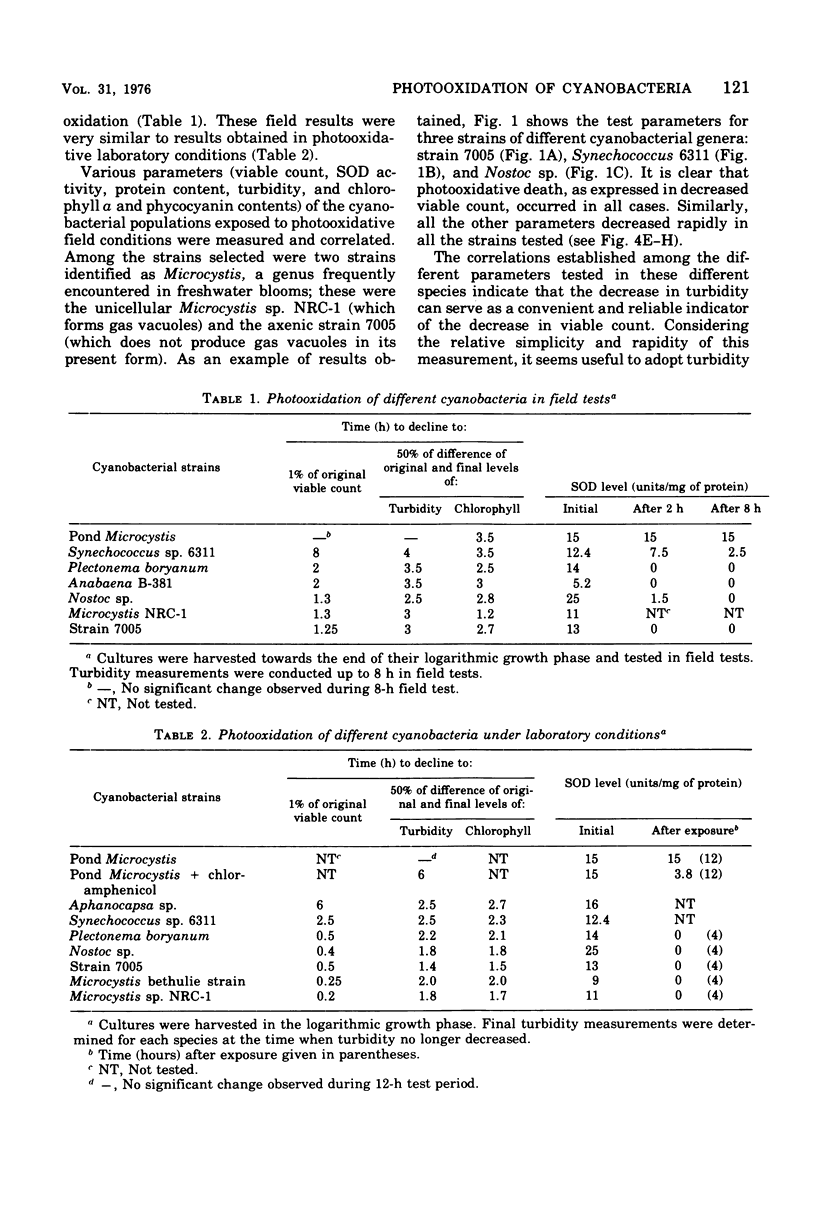
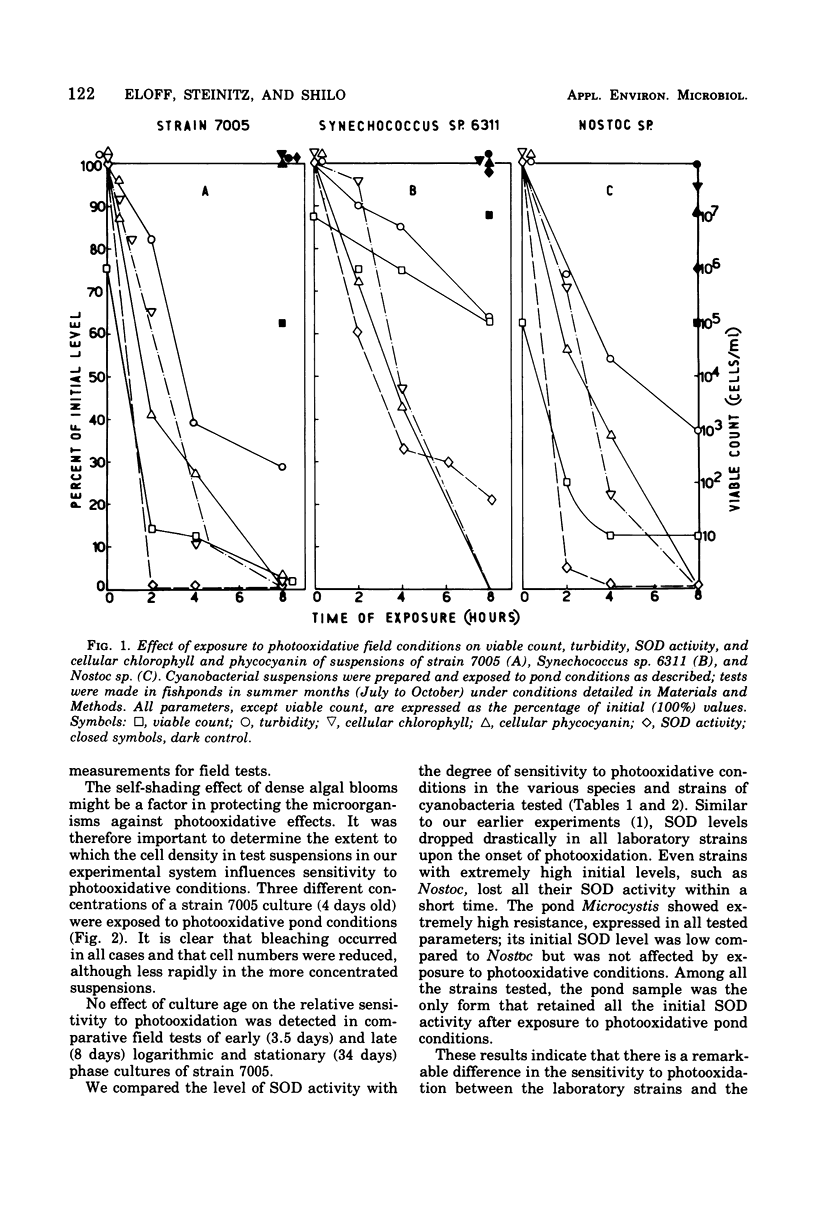
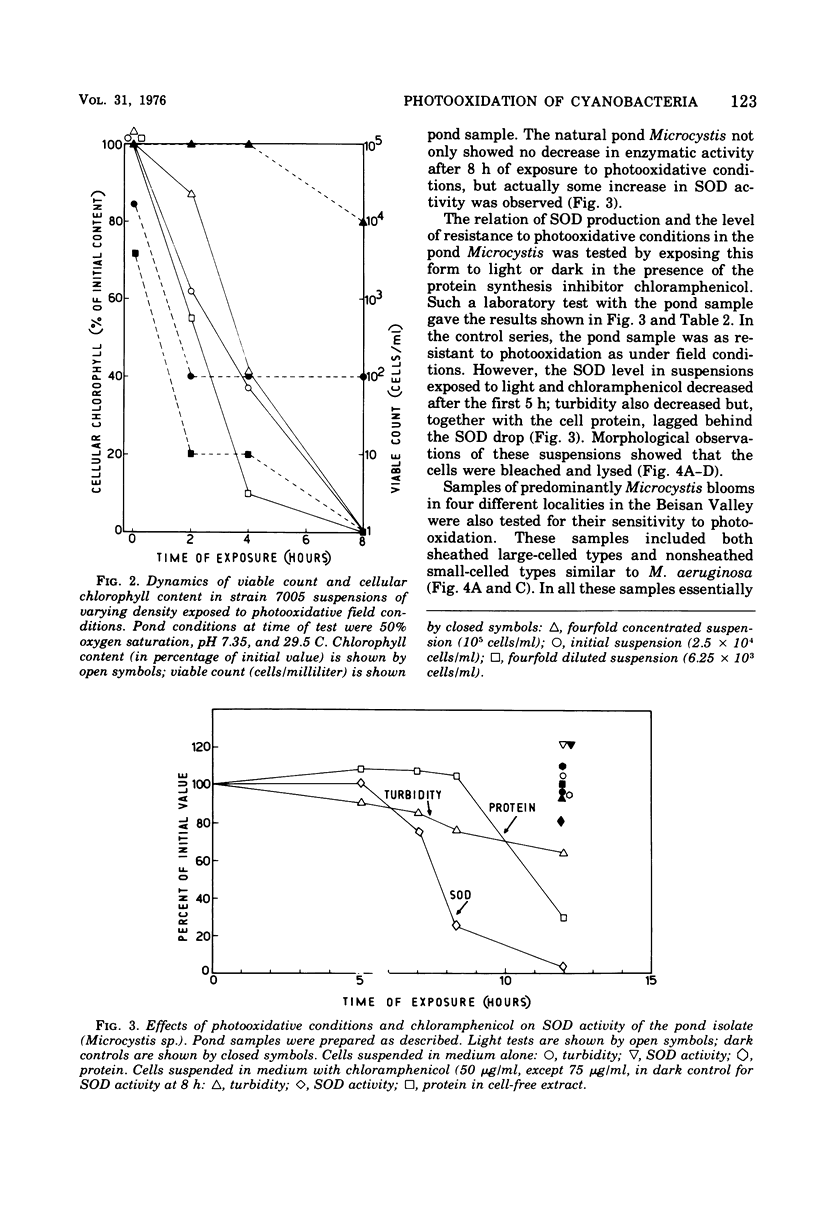
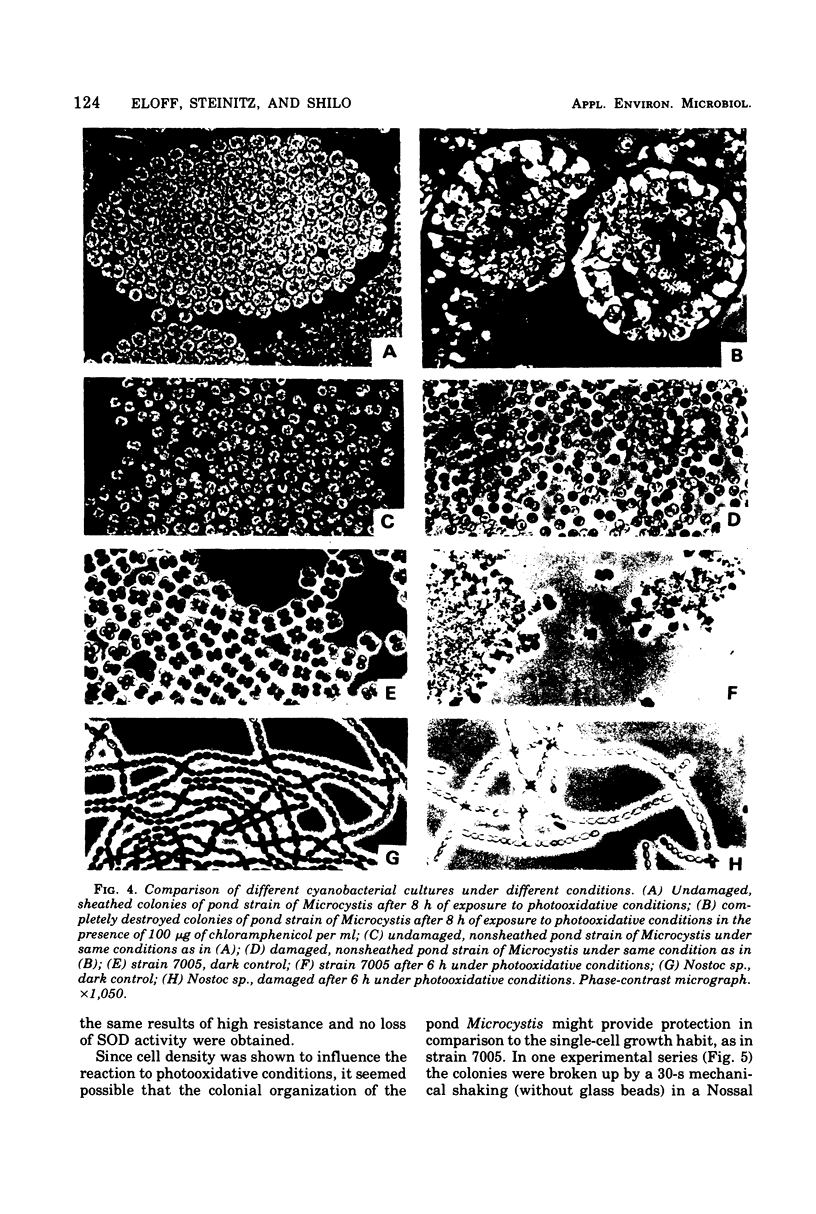
Photodynamic effects were demonstrated and assayed under field conditions in a number of different laboratory strains and pond isolates of cyanobacteria; parameters assayed for resistance to photooxidation were viable count, turbidity of the cyanobacterial suspension, and protein and pigment contents. The effects of density, colonial structure, and internal gas vacuoles on the lethal outcome were investigated. The stability and formation of superoxide dismutase under photooxidative conditions in the field and laboratory were studied in the different strains. An isolate of Microcystis from blooms in ponds exhibited extremely high resistance to photooxidation, which was abolished by exposure to chloramphenicol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Kellenberg D., Shilo M. Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol. 1974 May;19(5):379–382. doi: 10.1111/j.1751-1097.1974.tb06526.x. [DOI] [PubMed] [Google Scholar]
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The purification and properties of superoxide dismutase from Neurospora crassa. J Biol Chem. 1972 Jun 10;247(11):3410–3414. [PubMed] [Google Scholar]
- Padan E., Raboy B., Shilo M. Endogenous dark respiration of the blue-green alga, Plectonema boryanum. J Bacteriol. 1971 Apr;106(1):45–50. doi: 10.1128/jb.106.1.45-50.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA K., BENSON A. A., CALVIN M. The absorption spectra of suspensions of living micro-organisms. Biochim Biophys Acta. 1954 Dec;15(4):461–470. doi: 10.1016/0006-3002(54)90002-5. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]