Abstract
Recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors triggers an innate immune response to colonizing or invading bacteria. Conversely, many bacteria have evolved mechanisms to dampen this response by downregulating the synthesis of such PAMPs. We have previously demonstrated that Pseudomonas aeruginosa growing in mucopurulent human respiratory mucus from cystic fibrosis patients represses the expression of its flagellin, a potent stimulant of the innate immune response. Here we demonstrate that this phenomenon occurs in response to the presence of neutrophil elastase in such mucus. Nonpurulent mucus from animals had no such repressive effect. Furthermore, lysed neutrophils from human blood reproduced the flagellin-repressive effect ex mucus and, significantly, had no effect on the viability of this organism. Neutrophil elastase, a component of the innate host defense system, has been described to be bactericidal for gram-negative bacteria and to degrade bacterial virulence factors. Thus, the resistance of P. aeruginosa to the bactericidal effect of neutrophil elastase, as well as this organism's ability to sense this enzyme's presence and downregulate the synthesis of a PAMP, may be the key factors in allowing P. aeruginosa to colonize the lungs. These findings demonstrate the dynamic nature of this bacterium's response to host defenses that ensures its success as a colonizer and also highlights the dual nature of defense molecules that confer advantages and disadvantages to both hosts and pathogens.
Pseudomonas aeruginosa is an opportunistic pathogen of humans that infects immunocompromised patients, patients with cystic fibrosis (CF), and patients who are maintained on ventilators. CF is of particular interest in regard to P. aeruginosa because it is one of the few diseases where this organism persists in the lungs as a chronic colonizer before ultimately causing the death of such patients. Several plausible hypotheses have been proposed to explain the basis of the predilection of these patients for Pseudomonas infection, including the failure of defensin-mediated lung defense to clear this organism (32), increased adherence of bacteria to epithelial cells (1), and the failure of uptake of P. aeruginosa by airway cells due to defective CFTR-mediated uptake (11). A number of studies have shown that, in addition to these mechanisms that are believed to result from the underlying genetic defect, P. aeruginosa undergoes numerous changes during chronic colonization, which are believed to allow it to persist in the lungs despite a normal immune system. Chronically colonizing strains demonstrate numerous genetic and phenotypic changes, including alterations in the structure of their lipopolysaccharides (LPS) (9), conversion to a mucoid phenotype (7), and sometimes loss of their flagellum and pili (18). More recently, a longitudinal study has more precisely defined many of the genetic changes that may occur during prolonged colonization (23, 31).
In addition to the genetic changes that lead to new phenotypes, we and others have demonstrated that there are genetic events that occur when this organism is grown in mucopurulent secretions (mucus) from CF patients that do not occur in common laboratory media. One of the responses of particular interest is the rapid repression of flagellin synthesis (encoded by fliC), the principal component of its flagellum (28, 34). This phenomenon led us to hypothesize that infected mucus contains a specific factor to which P. aeruginosa responds by repressing the flagellin synthesis and thus dampen the host inflammatory response to this pathogen-associated molecular pattern (PAMP). Thus, the purpose of the present study was to elucidate the identity of the substance(s) in mucus that is responsible for this action. After examining a number of possible substances that are known to be present in purulent mucus, we concluded that the flagellin-repressive factor is inflammation derived rather than a normal mucus component. Specifically, neutrophil elastase (NE) was found to be the substance that represses the expression of fliC, and this effect was blocked by the addition of NE specific inhibitors. Furthermore, we found that even a high NE concentration (25 μM) had no effect on the viability and morphology of P. aeruginosa cells, which is in contrast to its effect on other gram-negative bacteria, where extremely low concentrations killed Escherichia coli (2, 3). Lastly, we demonstrate that the repression of fliC expression was independent of any action that NE may have on flagellin itself. These findings provide insight into the transcriptional regulation of one of the virulence genes by a host-derived signaling molecule during the pathogenesis of P. aeruginosa lung infections in CF patients.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. The construction of the fliC mutant and reporter strains was previously described (5, 34). The fliC mutant was transformed with a pDN19lacΩ plasmid containing the fliC promoter fused to a promoterless lacZ gene. Bacteria were grown at 37°C in M63 medium [15 mM (NH4)2SO4, 22 mM KH2PO4, 40 mM K2HPO4, 40 mM glucose, 1 mM MgSO4, 25 mM FeCl2]. Mucus samples from several CF patients were collected during their hospitalization at Shands Hospital at the University of Florida and stored at −20°C. The frozen mucus samples were subsequently thawed, pooled, sterilized by UV light treatment, dialyzed using a 10-kDa cutoff membrane against 1% sodium azide in M63 medium, and finally dialyzed against M63 medium alone. A mucus supernatant was prepared by centrifuging the pooled mucus samples at 40,000 rpm for 1 h at 4°C. This material was then diluted with M63 medium to give a 20% (vol/vol) concentration. Bacteria were grown in M63 medium, the 20% mucus supernatant (later referred to as mucus), or a 20% heat-inactivated mucus supernatant (referred to as heat-inactivated mucus) for 6 h. All bacterial concentrations were ascertained after growth to ensure that there were no differences in the number of cells used in Western blots. The addition of mucus to M63 medium did not significantly influence the bacterial growth rate (data not shown). Heat inactivation was performed by heating the mucus for 30 min at 70°C. The protein content of the mucus sample was measured, and the mucus was treated for specific experiments with pronase 1:40 (wt/wt) and incubated at 40°C for 2 h.
TABLE 1.
Bacterial strains and plasmid used in this study
| P. aeruginosa strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| PAK | Wild type | S. Lory |
| PAKΔfliC | PAK fliC mutant | 5 |
| PAKfliC::lacZ | PAK harboring pDN19lacΩ with fliC promoter fused to lacZ | 34 |
| PAKΔfliC::lacZ | PAK fliC mutant harboring pDN19lacΩ with fliC promoter fused to lacZ | This study |
| Plasmid | ||
| pDN19lacΩ | Promoterless lacZ oriV oriT; Tcr Strr Ω | 32a |
Tcr, tetracycline resistance; Strr, streptomycin resistance.
Western blot analysis.
Cultures were pelleted by centrifugation, denatured by boiling in sodium dodecyl sulfate sample buffer, and subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Prior to electrophoresis, equal numbers of cells were loaded into the wells as ensured by measuring the CFU and the optical density at 600 nm. The proteins were transferred to polyvinylidene difluoride membranes and finally probed using a polyclonal anti-flagellin antibody.
β-Galactosidase assay.
To measure transcription from the fliC promoter, we measured the β-galactosidase activity in P. aeruginosa PAKfliC::lacZ and its isogenic fliC mutant containing a PAKfliC::lacZ transcriptional fusion. Prior to assays, reporter strains were grown overnight in the M63 medium with or without mucus supernatant, diluted into the same medium supplemented with enzymes or inhibitors, and further grown for 6 h. The β-galactosidase activity was measured in triplicate as described previously (20).
Electron microscopy.
For transmission electron microscopy (TEM), bacteria were grown overnight in M63 and subcultured into different growth media with or without NE, as well as NE inhibitor, and allowed to grow under static conditions at 37°C for 24 h. A drop of each culture was applied on carbon-coated electron microscopy grids and, after a brief incubation, the grids were washed, and the attached bacteria were negatively stained with 1% phosphotungstic acid. Samples were examined with a Hitachi H-7000 transmission electron microscope.
Fractionation of mucus supernatant by using affinity column chromatography.
The mucus supernatant was fractionated by using HiTrap MonoQ column (Amersham Pharmacia). Briefly, the column was equilibrated with G buffer (5 mM Tris-HCl, 0.1 mM CaCl2 [pH 8.0]) at a 2-ml/min flow rate. A portion (3 ml) of mucus supernatant (15 mg/ml total protein) was loaded onto the equilibrated column at the same flow rate. Finally, proteins were eluted with G buffer and a KCl gradient from 10 to 100%. Depending upon the peak area, suitable fractions were pooled and concentrated by using Apollo 20 columns (Orbital Biosciences, Massachusetts). Bacteria were grown in 50% concentrated fractions. Western blot analysis was performed to examine the flagellin synthesis in different fractions as described above.
Determination of NE activity.
NE acitivty was determined spectrophotometrically using the specific substrate MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide (Calbiochem, La Jolla, CA) (21). Assays were performed at room temperature on 96-well plates. The rate of hydrolysis of the substrate was measured by adding 10 μl of the mucus supernatant fractions to 100 μl of a 1 mM substrate solution in 0.1 M HEPES buffer (pH 7.5) containing 0.5 M NaCl and 10% dimethyl sulfoxide. The increase in the absorption was measured continuously for 10 min at 410 nm in an automated recording spectrophotometer.
Neutrophil isolation.
Whole blood was collected from healthy volunteers. Informed consent was obtained from all subjects, and the study was conducted according to U.S. Department of Health and Human Services guidelines. Protocols were also approved by the University of Florida Institutional Review Board. Neutrophils were isolated from whole blood by using Ficoll-Hypaque gradient media (ICN Biomedicals, Irvine, CA), followed by the hypotonic lysis of contaminating red cells. The resulting neutrophil population was found to be 99% pure. Cells were suspended in RPMI medium without l-glutamine (Mediatech, Inc., Herndon, VA) and lysed with brief sonication.
Statistical analysis.
Where applicable, the results are expressed as the means ± the standard deviations (SD) of three independent experiments. A Student t test was used to compare mean values. A probability (P) of <0.05 was considered statistically significant.
RESULTS
Flagellin repression is caused by a heat-labile substance.
CF mucus is a highly complex mixture of substances that includes macromolecules derived from normal airway secretions, inflammation derived molecules, lipids, and cellular debris such as DNA (16). The inflammatory components include β-defensins, lactoferrin, serum components, antibacterial agents such as myeloperoxidase proteases, and antimicrobial peptides (13, 25, 29). It is also an iron- and sodium-limited environment because of higher activities of iron chelating agents and sodium reabsorption (15). We therefore sequentially and individually examined the effect of many of the above components of mucus on flagellin synthesis by adding them to a minimal medium (M63) or by manipulating their concentrations in the case of iron, magnesium, calcium, and sodium. None of the pure proteins such as defensins or lactoferrin that are available commercially reproduced the flagellin-repressive effect of mucus, nor did changing the cation concentrations of laboratory medium M63.
Purulent CF secretions also contain a mixture of heat-labile and heat-resistant molecules. To determine whether the flagellin-repressive signaling component was proteinaceous in nature, we supplemented the M63 medium with heat-inactivated mucus and mucus treated with pronase, a mixture of exo- and endo-proteinases that acts on peptide bonds. The growth of P. aeruginosa in heat-inactivated mucus (70°C for 30 min) and in unheated mucus treated with a mixture of proteinases resulted in the relief of mucus-mediated block in flagellin synthesis (Fig. 1A, lanes 3 and 4). A similar observation was reported by Palmer et al., who observed normal motility, implying the loss of the flagellum repression, after sputum was heated to 95°C prior to growth of P. aeruginosa (28). These data suggested that a heat-labile component, presumably a protein or glycoprotein, was responsible for the flagellin suppressive activity.
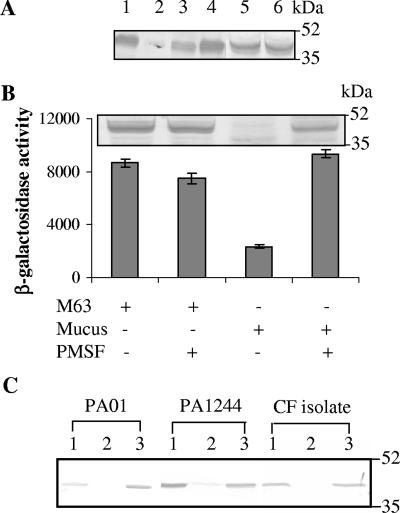
FIG. 1.
Flagellin repression is caused by a protein that is absent from animal mucus and is inhibited by a serine protease inhibitor. (A) Anti-flagellin immunoblot of P. aeruginosa grown for 6 h in M63 (lane 1), mucus (lane 2), heat-inactivated mucus (lane 3), pronase-treated mucus (lane 4), bovine submaxillary mucus (lane 5), and porcine gastric mucus (lane 6). In each experiment approximately equal numbers of bacteria based on the optical density at 600 nm and viable counts were used for the Western blots. Molecular mass markers are shown on the right in kilodaltons. (B) The addition of the serine protease inhibitor PMSF to mucus reestablished flagellin synthesis. The P. aeruginosa PAKfliC::lacZ reporter strain was grown either in M63 or in mucus with (+) or without (−) 1 mM PMSF for 6 h. The addition of mucus yielded a significant (P < 0.0005) reduction in fliC expression. On the other hand, the addition of PMSF to mucus significantly increased fliC transcription. Error bars indicate the means ± the SD. The values given were obtained from three independent experiments. The corresponding flagellin immunoblot is shown on the top. (C) The addition of PMSF reestablished flagellin synthesis in other P. aeruginosa strains. Lane 1, M63; lane 2, mucus; lane 3, mucus with PMSF.
Flagellin repression is caused by an inflammation-derived host component.
Among the proteins found in mucus, mucin glycoproteins make up the major component of mucus comprising up to 2% by weight of the mucus (6). To determine whether respiratory mucins were the repressive factor, we grew P. aeruginosa in medium containing mucins previously purified by ultracentrifugation as described previously (36), but this substance by itself had no repressive effect. However, mucopurulent CF secretions also contain large amounts of other proteins resulting from the inflammatory response to P. aeruginosa. If one of these proteins were responsible for flagellin repression, one would expect that mucus derived from sources that do not contain host-derived inflammatory products would be devoid of the flagellin-repressive activity. To test this hypothesis, commercially available bovine submaxillary mucus and porcine gastric mucus were tested for flagellin repression. Even at very high concentrations (up to 50%), neither type of mucus suppressed flagellin synthesis (Fig. 1A, lanes 5 and 6). Previous work by others (28) also indicated that neither mucus collected from primary cultures of lung epithelial cells nor bovine submaxillary mucin blocked the motility of P. aeruginosa. Taken together, these results suggest that the repressive component may be inflammation derived rather than a normal mucus constituent.
Serine proteases are responsible for the repression of flagellin synthesis.
In addition to defensins, lactoferrin, and cytokines that are released during inflammation, other inflammatory components include substantial numbers of polymorphonuclear leukocytes and the products they release, which include an array of antibacterial proteases. To investigate whether neutral serine proteases, which are abundant in neutrophil granules, were responsible for the repression of P. aeruginosa flagellin synthesis, we grew P. aeruginosa in mucus supplemented with various protease inhibitors. The addition of 1 mM phenylmethylsulfonyl fluoride (PMSF), a group-specific serine protease inhibitor, to cultures growing in purulent mucus reversed the repression of flagellin synthesis compared to cultures grown in mucus alone (Fig. 1B, lane 4). Serine proteases have been shown to degrade flagellin purified from P. aeruginosa and Salmonella enterica serovar Typhimurium (17). However, this was unlikely to be the explanation in our case since it was previously demonstrated that during growth in mucus the repressive effect on flagellin synthesis takes place at the transcriptional level (34). Therefore, the transcription of the P. aeruginosa fliC gene was examined in mucus containing exogenous PMSF by using a reporter strain carrying the fliC promoter fused to a promoterless lacZ gene. Under these conditions, the fliC promoter activity increased ∼5-fold (P < 0.0005) compared to that of cultures grown in mucus alone (Fig. 1B). PMSF alone had no repressive effect on flagellin synthesis in M63 medium (Fig. 1B), indicating that a serine protease(s) was responsible for the repression of flagellin synthesis in mucus. Similar observations were made in P. aeruginosa strains PAO1, PA1244, and CF0202509 (a CF isolate) that flagellin synthesis was repressed in mucus and reestablished in the presence of PMSF (Fig. 1C). These results suggest that the response to serine protease(s) appears to be generalized among Pseudomonas strains.
NE represses flagellin synthesis.
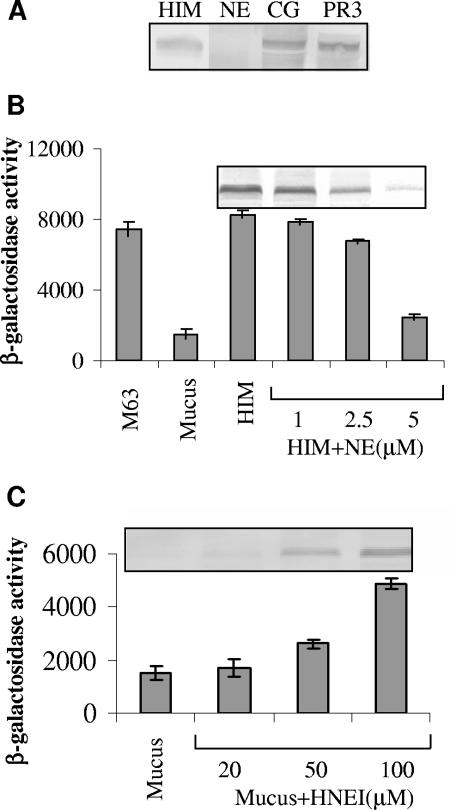
Upon activation, neutrophils release predominantly three neutrophil serine proteases—NE, cathepsin G (CG), and proteinase 3 (PR3)—from azurophilic granules (26). To determine the specific serine protease responsible for flagellin repression, P. aeruginosa was grown in 20% heat-inactivated mucus in the presence or absence of these serine proteases (5 μM each). Western immunoblots indicated that only purified human NE repressed flagellin synthesis at the doses used (Fig. 2A), suggesting that NE is probably the protease responsible for the observed phenomenon. This decrease in flagellin synthesis was NE concentration dependent. As shown in Fig. 2B (top), a substantial reduction in flagellin synthesis was observed with increasing NE concentration (from 1 to 5 μM). In order to ascertain whether NE itself replicates the same effect seen in mucus, the transcription of fliC was examined by growing P. aeruginosa PAKfliC::lacZ reporter strain in heat-inactivated mucus with or without added proteases. We observed an ∼4.0-fold decrease (P < 0.0005) in fliC transcription after the addition of 5 μM NE to the heat-inactivated mucus (Fig. 2B). The concentrations of NE used in these experiments are in the range of those present in the epithelial lining fluid (ca. 8 μM) of CF patients (19). In contrast to NE, both CG and PR3 used at the same concentration had no effect on fliC transcription (data not shown). These results extend the perception that NE is explicitly responsible for this phenomenon. Surprisingly, none of these proteases had an effect on the viability of P. aeruginosa, indicating that the decrease in flagellin level is not due to a reduction in cell numbers.
FIG. 2.
Effect of human neutrophil proteases on flagellin synthesis. (A) NE altered flagellin expression. P. aeruginosa PAK was incubated in heat-inactivated mucus (HIM) with or without NE, CG, and PR3 (5 μM) for 6 h. The reactions were processed for immunoblotting with flagellin-specific antibody. Similar numbers of bacteria were loaded, as ensured by measuring the CFU. (B) Human NE repressed fliC transcription. The activity of the fliC promoter was measured by growing the P. aeruginosa PAKfliC::lacZ reporter strain in M63, mucus, or heat-inactivated mucus (HIM) alone or in the presence of HIM supplemented with different concentrations of NE (1, 2.5, and 5 μM) for 6 h. The addition of 5 μM NE significantly (P < 0.0005) decreased fliC expression. Error bars indicate the means ± the SD. The values given were obtained from three independent experiments. The corresponding Western blots with flagellin-specific antibody are shown at the top of the graph. (C) The inhibition of NE activity by HNEI increased fliC expression. The reporter strain was incubated in mucus alone and in mucus containing different concentrations of HNEI (20, 50, and 100 μM) for 6 h. At 100 μM HNEI, a substantial increase in fliC expression was observed (P < 0.0005). Error bars indicate the means ± the SD. The corresponding immunoblot is shown at the top.
NE has been regarded as an antibacterial protein whose catalytic activity relies on the His-Asp-Ser triad, where Ser is the active residue (39). To date, several NE inhibitors have been described. In the present study, we used the peptidyl human NE inhibitor methoxysuccinyl-Ala-Ala-Pro-Ala chloromethyl ketone (HNEI; MeOSuc-AAPA-cmk) (22). HNEI has been shown to be one of the most commonly used NE specific inhibitors that inhibits NE activity by cross-linking the catalytic residues His-57 and Ser-195 of the active site. We presumed that the inhibition of NE activity should release the block in fliC transcription. To test this hypothesis, we grew P. aeruginosa in mucus supplemented with different doses of HNEI. The addition of HNEI to mucus, increased flagellin transcription (Fig. 2C, bottom) and translation (Fig. 2C, top) in a dose-dependent manner, such that the addition of 100 μM HNEI resulted in an ∼4.0-fold increase (P < 0.0005) in the fliC transcription compared to mucus alone. This corresponds well to the results obtained with 5 μM NE (Fig. 2B).
NE containing mucus fraction exhibited flagellin-repressive activity.
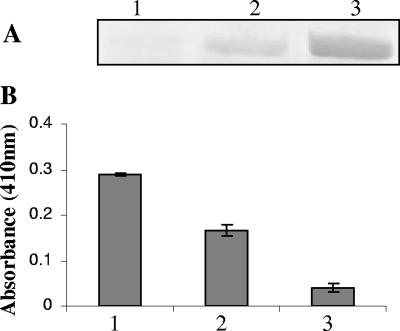
In an alternative approach to implicate the involvement of NE, mucus supernatant was fractionated by passage through a HiTrap MonoQ column (see Materials and Methods). Approximately 15 to 20 fractions of 1 ml each were collected and then pooled into 4 to 5 fractions depending upon the protein peaks. Finally, the pooled fractions were concentrated about 10 times. Incubation of P. aeruginosa in the pooled fractions resulted in the complete repression of flagellin expression in one of the fractions, partial repression in another adjacent fraction, and no repression in the remaining fractions (Fig. 3A). NE activity was measured in these pooled fractions and demonstrated an approximately 60% higher activity in the repressing fraction than in the partially repressing fraction, whereas no activity was detected in the nonrepressive fraction (Fig. 3B). Taken together, these results strongly indicate that human NE present in mucopurulent CF airway secretions is responsible for the repression of fliC of P. aeruginosa.
FIG. 3.
NE activity in different mucus fractions. Mucus was fractionated by using a HiTrap MonoQ column as described in Materials and Methods. P. aeruginosa PAK was grown in completely repressing, partially repressing, and nonrepressing fractions. (A) Western blot was performed with flagellin-specific antibody. (B) NE activity was measured in fractions 1 to 3 (see Materials and Methods). Fractions: 1, completely repressing; 2, partially repressing; 3, nonrepressing.
NE requires a specific environment to remain active.
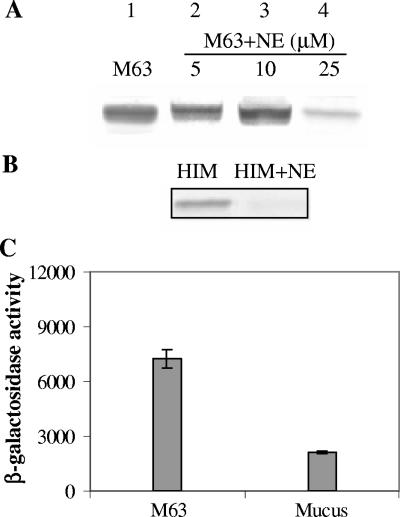
Enzymes often need specific environments to remain reasonably active. A number of enzymes require cofactors or specific substrates to remain in an active state. For example, the degradation of surfactant protein D by NE is dependent upon the calcium ion concentration (12). CF mucus is very complex in nature and contains a large number of degradation products and substrates that could enhance the NE activity. In light of this, we compared the NE activity in mucus to that in M63 medium. For these experiments, we used NE concentrations ranging from 5 to 25 μM. In M63, flagellin repression occurred only at an extremely high NE concentration (25 μM); this was five times greater than the concentration we used in other experiments (Fig. 4A). On the other hand, the addition of a very low amount of heat-inactivated mucus (2%), which had no suppressive activity on its own, to M63 containing 5 μM NE abolished flagellin synthesis (Fig. 4B), suggesting that NE is more active in the mucus environment. These data suggest that any potential coeffector molecule(s) is not a dialyzable low-molecular-weight compound or another protein since heat-treated, dialyzed mucus was used in these studies. Significantly, P. aeruginosa was not killed at a 25 μM NE concentration in M63, implying that even at this supraphysiological concentration NE is not bactericidal for this strain of P. aeruginosa. Similarly, P. aeruginosa strain PAO1 remained viable at 25 μM NE. Furthermore, at 5 μM NE the morphology of P. aeruginosa appeared normal (data not shown).
FIG. 4.
The flagellin-repressive effect of NE is greater in a mucus environment and is not mediated through flagellin degradation. (A) P. aeruginosa PAK was grown under different conditions. Lane 1, M63 alone; lanes 2, 3, and 4, M63 containing 5, 10, and 25 μM NE, respectively. Immunoblotting was performed with anti-flagellin antibody. (B) Immunoblot with or without NE. Lane 1, M63 containing 2% heat-inactivated mucus (HIM); lane 2, M63 containing 2% heat-inactivated mucus and 5 μM NE. (C) P. aeruginosa fliC mutant containing fliC-lacZ reporter was grown in M63 and mucus for 6 h. However, the fliC expression was still repressed (P < 0.0005) after growth in mucus. Error bars indicate the means ± the SD. The values given are the means of three independent experiments.
Repression of fliC transcription is not mediated through flagellin degradation.
NE has the ability to degrade virulence factors and surface-exposed proteins of enterobacteria (3, 33). It has previously been reported that NE targeted the virulence proteins secreted by Salmonella and Yersinia. The Salmonella virulence proteins SipA, SipB, SipC, and HAPs, required for flagellar structure, and the Yersinia proteins YopB, YopD, and YopE were degraded by NE (33). Thus, it is quite possible that the signal that mediates the expression of fliC is transmitted through flagellin degradation. Therefore, to examine whether the mechanism of fliC repression in mucus involves flagellin degradation, the activity of the fliC promoter fused to a promoterless lacZ gene was examined by growing PAK-fliC deletion mutant in absence or presence of mucus. However, similar to the wild-type strain, the fliC promoter of the mutant remained repressed (Fig. 4C).
Neutrophil cell extract exhibits flagellin-repressive activity.
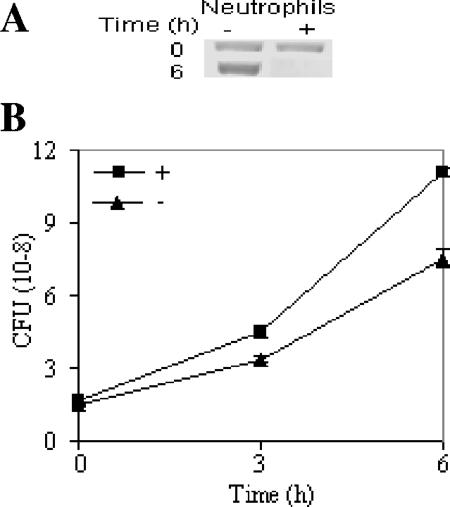
Elastase is secreted mainly by neutrophils and macrophages. To ascertain whether these observations are relevant in vivo and that flagellin repression occurs due to a neutrophil derived product, we assessed flagellin expression after incubating P. aeruginosa with a human neutrophil lysate (1.6 × 107 cells/ml) enriched in granule proteins. Flagellin synthesis was markedly reduced in the culture grown in the presence of the neutrophil lysate (Fig. 5A). Since neutrophils release a large number of antibacterial substances, we also determined the effect of neutrophil lysate on bacterial viability. However, the lysed neutrophils at this concentration had no significant effect on the growth of P. aeruginosa (Fig. 5B). Since the lysate had no effect on the viability or morphology of this organism, it is unlikely that the decrease in flagellin was the result of the degradation of both intracellular and extracellular flagellin.
FIG. 5.
Neutrophil lysate in tissue culture medium also repressed flagellin synthesis. P. aeruginosa PAK was grown in the presence (+) or absence (−) of neutrophil lysate for 6 h. Samples were removed at different time intervals (0, 3, and 6 h), and the reactions were processed for immunoblotting (A) and CFU counts (B). Western blotting was performed with flagellin-specific antibody.
NE was responsible for blockage in the synthesis of surface appendages.
Using TEM, we found morphological evidence that the absence of assembled flagellar structures was due to NE activity. The organisms were unable to form these appendages in mucus alone and in heat-inactivated mucus supplemented with 5 μM NE (Fig. 6B), whereas the same structures were evident on bacteria grown in mucus supplemented with HNEI and in heat-inactivated mucus (Fig. 6C and D). Depending upon the conditions used, between 80 and 95% cells were either flagellated or nonflagellated. We also performed a microscopic examination of cell motility at different time intervals after the cells were exposed to mucopurulent mucus. Approximately 90% cells became nonmotile after 3 h. This is probably due to two actions: the degradation of flagellin by NE (17) and the cessation of flagellin transcription within 4 h, as we have noted previously (34).
FIG. 6.
TEM of P. aeruginosa strain PAK in M63 (A) and in heat-inactivated mucus (2%) containing 5 μM NE and mucus (B). Since in both conditions, i.e., in mucus alone and in 2% heat-inactivated mucus supplemented with 5 μM NE, cells were nonflagellated, only representative cells are shown. Mucus with 100 μM HNEI (C) and heat-inactivated mucus (D). Scale bar, 1 μ.
DISCUSSION
CF is characterized by chronic airway colonization, primarily due to P. aeruginosa, that causes cycles of lung inflammation and leads to a decline in lung function, respiratory failure, and subsequently to death. During chronic colonization the organism demonstrates a number of responses to life in the CF airway that may allow it to become adapted to this inflammatory environment and be established as a chronic colonizer (23). These changes appear to result from mutational events and the subsequent selection for traits, which may confer survival advantages. However, such changes occur over a prolonged period of time and do not explain the early failure of innate immune mechanisms to prevent colonization. On the other hand, the changes that are noted in gene expression when this organism is grown in mucopurulent secretions (28, 34) may shed light on the mechanisms underlying this failure. Based on these observations, we suggested that the suppression of flagellin synthesis, which is observed within 4 h of growing the organism in mucus (34), may be a means of evading innate immunity by this organism. Nevertheless, it may be argued that P. aeruginosa possesses another major PAMP, LPS, which has the potential to stimulate a defensive innate response (8, 9) in case of loss of flagellin; thus, there may be a secondary means of stimulating an innate immune response. However, it now appears that flagellin is likely to be the major PAMP to initiate a defensive reaction by airway cells, since LPS, the other major Toll-like receptor agonist, is poorly recognized by respiratory airway cells (14, 38). Therefore, the suppression of flagellin synthesis may be a critical event, allowing P. aeruginosa to evade an innate immune response in the early stages of an airway infection.
The present study provides evidence that suppression of flagellin synthesis results from the action of a host-derived inflammatory product rather than a normal mucus constituent. The data show that among the three active neutrophil serine proteases, NE is at least one of the molecules responsible for promoting a genetic and phenotypic change that may provide survival advantages to the pathogen. We demonstrate that NE specifically modulates the expression of flagellin at both the transcriptional and the translational levels, and this effect was prevented when the catalytic activity of the enzyme was inhibited by using specific inhibitors. The central role of NE in this dynamic phenomenon was further shown by TEM, mucus fractions containing high NE activities, and microscopic examination of live bacteria for motility. It is unlikely that this is a generalized stress response since this does not occur when P. aeruginosa is confronted with reactive oxygen radicals (27). In a similar paradigm involving the response of this organism to host molecules, P. aeruginosa has been shown to sense the presence of gamma interferon, an innate immune effector molecule (37). Wu et al. showed that gamma interferon binds to the P. aeruginosa surface protein OprF, resulting in the upregulation of intracellular lectin PA-I (lecA). PA-I is known to induce apoptosis in respiratory epithelial cells. Thus, we hypothesize that NE could function by degrading surface proteins to which the organism responds by triggering the flagellin-repressive effect. However, repression of the flagellin promoter occurs even in a fliC mutant; therefore, if the mechanism of flagellin suppression involves degradation, it is likely that it goes through the degradation of protein(s) other than flagellin itself. It is difficult to explain how P. aeurignosa, which is primarily an environmental organism, shows such a response to human NE. It is quite possible that the proteins with significant similarity to NE might also be present in the environment or that this is purely accidental but proves to be of benefit to the organism.
The in vivo relevance of these findings was further demonstrated using neutrophils isolated from a healthy individual's blood. Neutrophils release a large number of substances that kill bacteria. We observed that bacterial killing was deficient under conditions in which Pseudomonas cells were incubated with neutrophil lysate. This was further proven by the observation that P. aeruginosa cells were intact and viable even at extremely high NE concentrations (5 to 25 μM). This is in contrast to previous findings, which indicated that 2 μM NE killed E. coli and also perturbed its morphology (3). This finding could be of biological significance that may partly explain how Pseudomonas cells persist in the CF lung environment despite the massive influx of neutrophils. In addition to the CF lung, several studies have demonstrated increased neutrophil counts and thus NE activity in human lungs in cases of hospital-acquired pneumonia (4, 30).
Thus, the observation presented here that flagellin is suppressed by NE could elucidate how and why CF patients undergo cyclical exacerbations of the inflammatory lung disease caused by P. aeruginosa. When neutrophil numbers and thus NE concentrations are low, P. aeruginosa may proliferate, assemble a flagellum, and release flagellin, stimulating a robust inflammatory response (10). Neutrophil numbers and NE concentrations would increase in response to flagellin and, when they reach high enough levels, flagellin synthesis may be suppressed, bacterial counts will fall, and flagellin-mediated inflammation may then abate. When the numbers of neutrophils and the concentration of NE decrease enough, flagellin synthesis resumes, and there is a resurgence of inflammation. Thus, such cycles may play a role in exacerbations of inflammation that are independent of the ongoing activity of any other inflammatory factor, especially early in this disease when nonmucoid bacteria are present in the airways.
A number of cogent arguments have already been proposed for the inhibition of NE activity in CF patients (24). The observations made here further support such an approach since the inhibition of the NE activity early in this disease may allow the generation of a more sustained innate immune response to P. aeruginosa, while preventing the damage caused by NE. If the interpretation of our findings is correct, a corollary would be that inhibition of NE should result in a more sustained innate immune response to flagellin and possibly in better clearance of P. aeruginosa from the lungs of such individuals. This observation has recently been made using an animal model (35) and deserves further study.
Acknowledgments
We thank S. Jin, University of Florida, Gainesville, for critical reading of the manuscript, and A. Verma, our laboratory colleague for discussions.
This study was supported by a grant R01-AI45014 from the National Institutes of Health to R.R.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Adamo, R., S. Sokol, G. Soong, M. I. Gomez, and A. Prince. 2004. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30:627-634. [DOI] [PubMed] [Google Scholar]
- 2.Belaaouaj, A. 2002. Neutrophil elastase-mediated killing of bacteria: lessons from targeted mutagenesis. Microbes Infect. 4:1259-1264. [DOI] [PubMed] [Google Scholar]
- 3.Belaaouaj, A., K. S. Kim, and S. D. Shapiro. 2000. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 289:1185-1188. [DOI] [PubMed] [Google Scholar]
- 4.Boutten, A., M. S. Dehoux, N. Seta, J. Ostinelli, P. Venembre, B. Crestani, M. C. Dombret, G. Durand, and M. Aubier. 1996. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 153:336-342. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 6.Davies, J. R., A. Herrmann, W. Russell, N. Svitacheva, C. Wickström, and I. Carlstedt. 2002. Respiratory tract mucins: structure and expression patterns, p. 76-93. In Novartis Foundation Symposium 248: Mucus Hypersecretion in Respiratory Disease. John Wiley & Sons, Chichester, United Kingdom. [PubMed]
- 7.Doggett, R. G. 1969. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl. Microbiol. 18:936-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, R. K., A. M. Hajjar, J. H. Tsai, S. M. Moskowitz, C. B. Wilson, and S. I. Miller. 2003. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J. Endotoxin. Res. 9:395-400. [DOI] [PubMed] [Google Scholar]
- 9.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 10.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg, J. B., and G. B. Pier. 2000. The role of the CFTR in susceptibility to Pseudomonas aeruginosa infections in cystic fibrosis. Trends Microbiol. 8:514-520. [DOI] [PubMed] [Google Scholar]
- 12.Hircher, T. O., E. C. Crouch, M. Espinola, T. J. Brokelman, R. P. Mecham, N. DeSilva, J. Cooley, E. Remold-O'Donnell, and A. Belaaouaj. 2004. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J. Biol. Chem. 279:27688-27698. [DOI] [PubMed] [Google Scholar]
- 13.Hoiby, N. 1998. Pseudomonas in cystic fibrosis: past, present, and future. Cystic Fibrosis Trust, London, United Kingdom.
- 14.Hybiske, K., J. K. Ichikawa, V. Huang, S. J. Lory, and T. E. Machen. 2004. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell Microbiol. 6:49-63. [DOI] [PubMed] [Google Scholar]
- 15.Knowles, M. R., and R. C. Boucher. 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 109:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillehoj, E. R., and K. C. Kim. 2002. Airway mucus: its components and function. Arch. Pharm. Res. 25:770-780. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Boado, Y. S., M. Espinola, S. Bahr, and A. Belaaouaj. 2004. Neutrophil serine proteinases cleave bacterial flagellin, abrogating its host response-inducing activity. J. Immunol. 172:509-515. [DOI] [PubMed] [Google Scholar]
- 18.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElvaney, N. G., R. C. Hubbard, P. Birrer, M. S. Chernick, D. B. Caplan, M. M. Frank, and R. G. Crystal. 1991. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet 337:392-394. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Nakajima, K., J. C. Powers, B. M. Ashe, and M. Zimmerman. 1979. Mapping the extended substrate-binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J. Biol. Chem. 254:4027-4032. [PubMed] [Google Scholar]
- 22.Navia, M. A., B. M. McKeever, J. P. Springer, T. Y. Lin, H. R. Williams, E. M. Fluder, C. P. Dorn, and K. Hoogsteen. 1989. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-Å resolution. Proc. Natl. Acad. Sci. USA 86:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen, D., and P. K. Singh. 2006. Evolving stealth: genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc. Natl. Acad. Sci. USA 103:8305-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohbayashi, H. 2002. Novel neutrophil elastase inhibitors as a treatment for neutrophil-predominant inflammatory lung diseases. IDrugs 5:910-923. [PubMed] [Google Scholar]
- 25.Ohman, D. E., and A. M. Chakrabarty. 1982. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect. Immun. 37:662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen, C. A., and E. J. Campbell. 1999. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 65:137-150. [DOI] [PubMed] [Google Scholar]
- 27.Palma, M., D. DeLuca, S. Worgall, and L. E. Quadri. 2004. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 186:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer, K. L., M. L. Mashburn, P. K. Singh, and M. Whiteley. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187:5267-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Vilar, J., and R. C. Boucher. 2004. Reevaluating gel-forming mucins' roles in cystic fibrosis lung disease. Free Radical Biol. Med. 37:1564-1577. [DOI] [PubMed] [Google Scholar]
- 30.Schaff, B., A. Wieghorst, S.-P. Aries, K. Dalhoff, and J. Braun. 2000. Neutrophil inflammation and activation in bronchiectasis: comparison with pneumonia and idiopathic pulmonary fibrosis. Respiration 67:52-59. [DOI] [PubMed] [Google Scholar]
- 31.Smith, E. E., D. G. Buckley, Z. Wu, C. Saenphimmachak, L. R. Hoffman, D. A. D'Argenio, S. I. Miller, B. W. Ramsey, D. P. Speert, S. M. Moskowitz, J. L. Burns, R. Kaul, and M. V. Olson. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 103:8487-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, J. J., S. M. Travis, E. P. Greenberg, and M. J. Welsh. 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85:229-236. [DOI] [PubMed] [Google Scholar]
- 32a.Totten, P. A., and S. Lory. 1990. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J. Bacteriol. 172:7188-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinrauch, Y., D. Drujan, S. D. Shapiro, J. Weiss, and A. Zychlinsky. 2002. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417:91-94. [DOI] [PubMed] [Google Scholar]
- 34.Wolfgang, M. C., J. Jyot, A. L. Goodman, R. Ramphal, and S. Lory. 2004. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 27:6664-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods, D. E., A. Cantin, J. Cooley, D. M. Kenney, and E. Remold-O'Donnell. 2005. Aerosol treatment with MNEI suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Pediatr. Pulmonol. 39:141-149. [DOI] [PubMed] [Google Scholar]
- 36.Woodward, H., B. Horsey, V. P. Bhavanandan, and E. A. Davidson. 1982. Isolation, purification, and properties of respiratory glycoproteins. Biochemistry 21:694-701. [DOI] [PubMed] [Google Scholar]
- 37.Wu, L., O. Estarda, O. Zaborina, M. Bains, L. Shen, J. E. Kohler, N. Patel, M. W. Musch, E. B. Chang, Y. X. Fu, M. A. Jacobs, M. I. Nishimura, R. E. Hancock, J. R. Turner, and J. C. Alverdy. 2005. Recognition of host immune activation by Pseudomonas aeruginosa. Science 309:774-777. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Z., J. P. Loboutin, D. J. Weiner, J. B. Goldberg, and J. M. Wilson. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 73:7151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmer, M., R. L. Medcalf, T. M. Fink, C. Mattmann, P. Lichter, and D. E. Jenne. 1992. Three human elastase-like genes coordinately expressed in the myelomonocyte lineage are organized as a single genetic locus on 19pter. Proc. Natl. Acad. Sci. USA 89:8215-8219. [DOI] [PMC free article] [PubMed] [Google Scholar]