Abstract
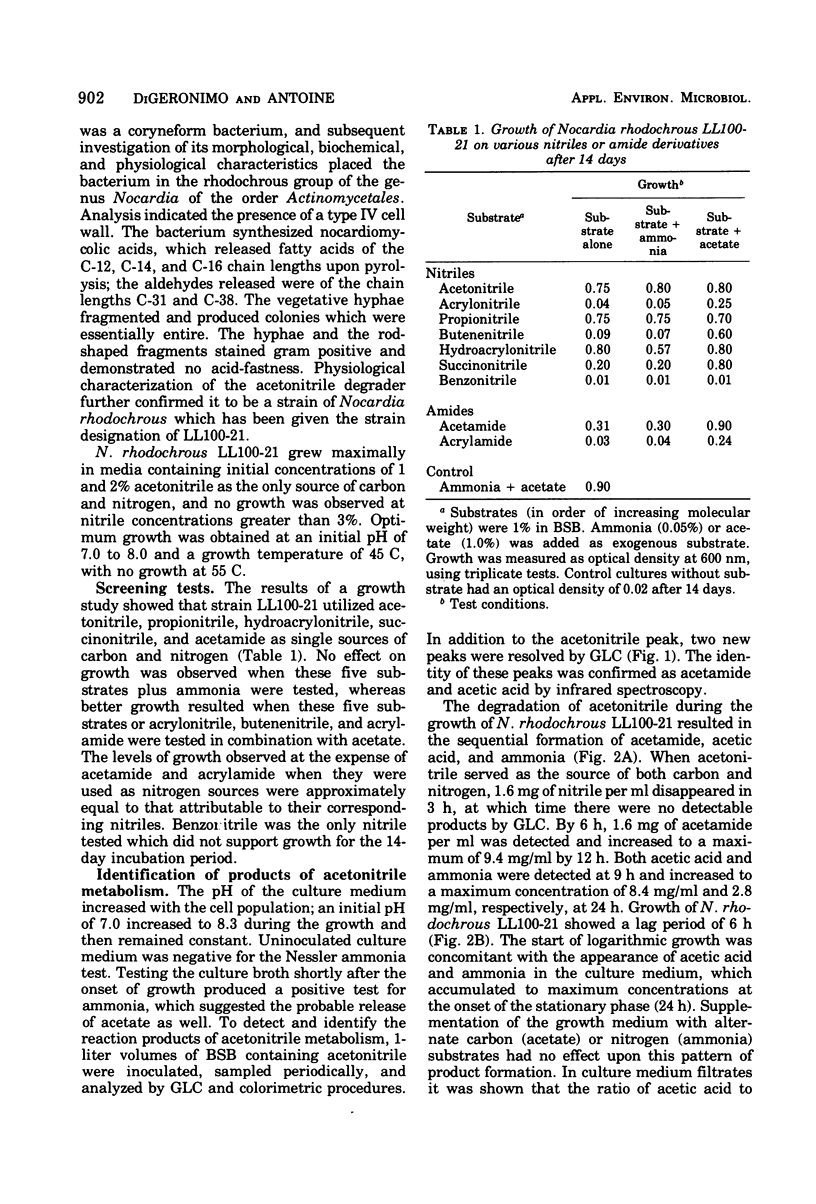
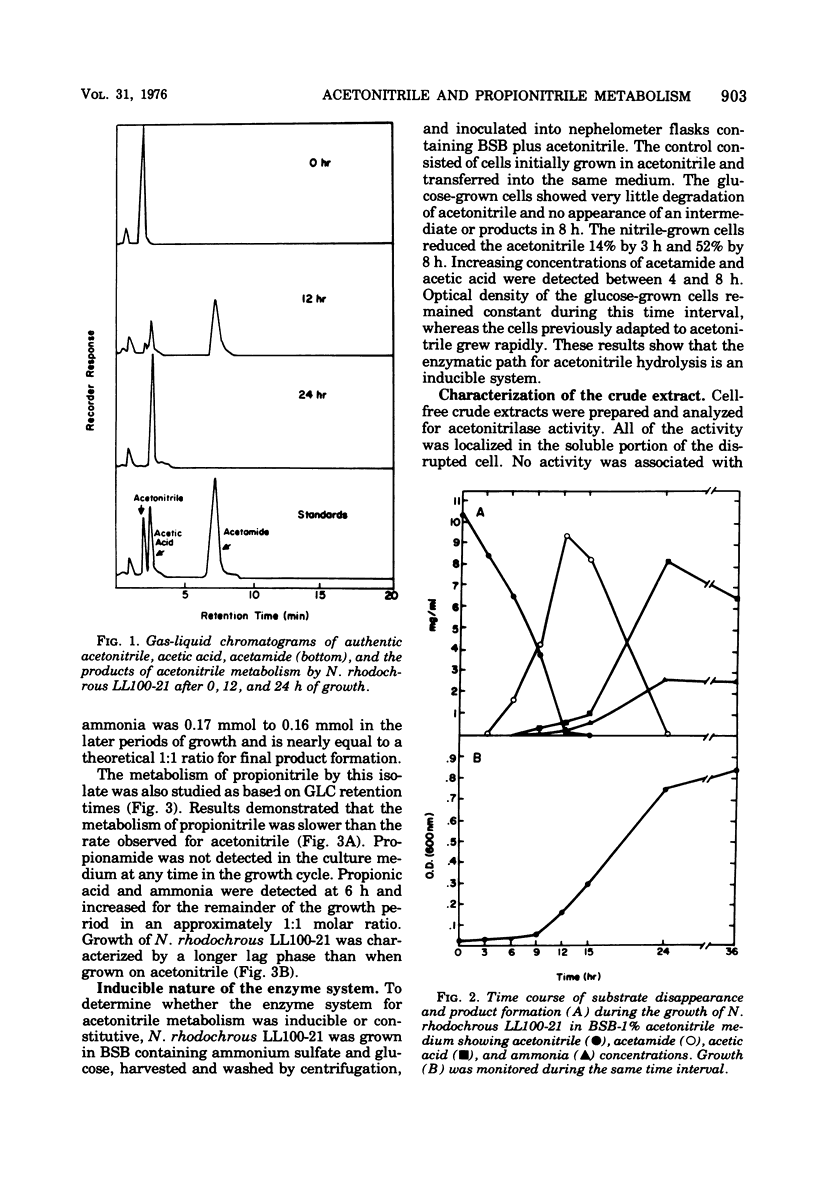
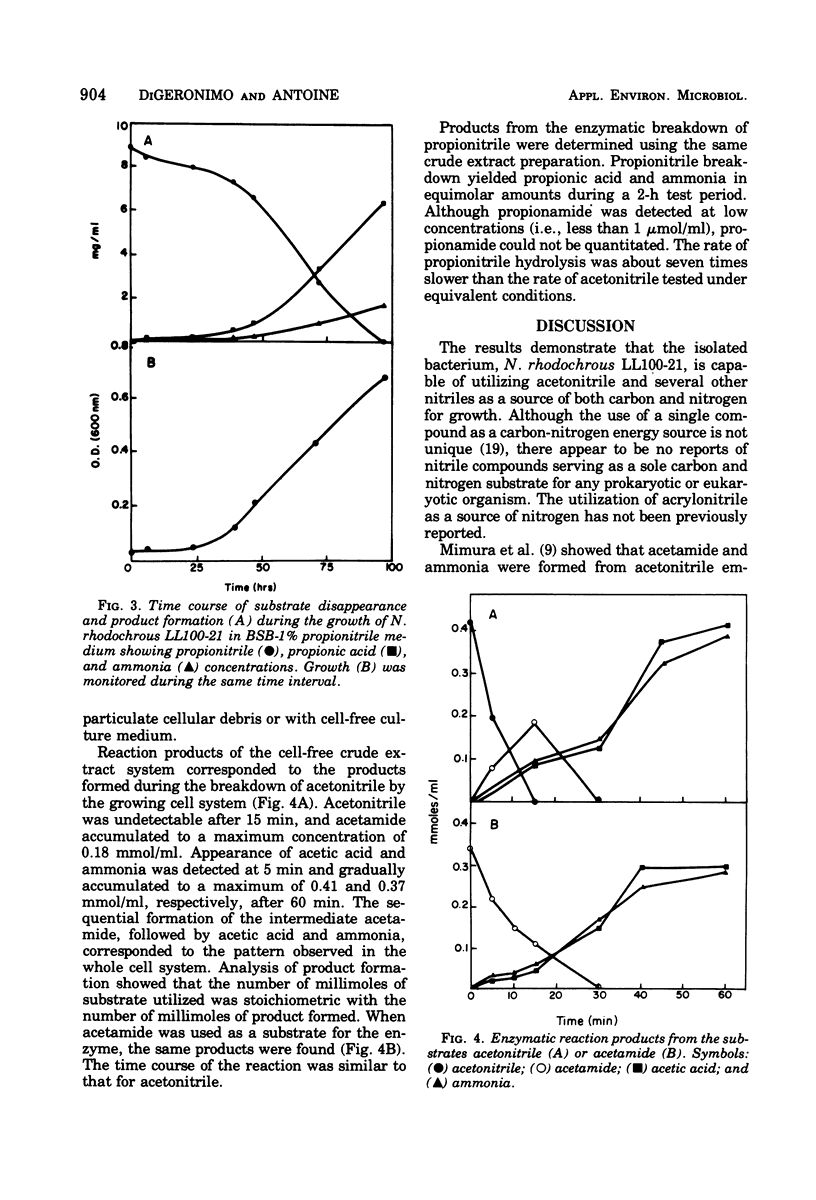
Six nitrile compounds and two amide derivatives were degraded by Nocardia rhodochrous LL100-21. Acetonitrile, hydroacrylonitrile, and propionitrile were the best sources of carbon and nitrogen for growth, whereas butenenitrile, succinonitrile, and acetamide supported less growth. Acrylonitrile and acrylamide supported growth but only as a source of nitrogen. Gas chromatography of the culture medium revealed a decrease in acetonitrile with the sequential formation of acetamide and acetic acid. Ammonia was also detected by colorimetric procedures. The enzyme system responsible for the hydrolysis of acetonitrile was shown to be intracellular and inducible. The breakdown of acetonitrile by a crude bacterial extract was a two-step enzymatic hydrolysis with acetamide as the intermediate product and acetic acid and ammonia as the final products. Product formation was stoichiometric with substrate disappearance. When propionitrile was the growth substrate, there was complete conversion of the nitrile to propionic acid and ammonia as the major products. The enzymatic breakdown of the propionitrile, although slower than acetonitrile, yielded the corresponding carboxylic acid and ammonia. Propionamide was produced in very small amounts as an intermediate product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon R. E. Some criteria for the recognition of Nocardia madurae (Vincent) Blanchard. J Gen Microbiol. 1966 Nov;45(2):355–364. doi: 10.1099/00221287-45-2-355. [DOI] [PubMed] [Google Scholar]
- HOOK R. H., ROBINSON W. G. RICININE NITRILASE. II. PURIFICATION AND PROPERTIES. J Biol Chem. 1964 Dec;239:4263–4267. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lechevalier M. P., Lechevalier H., Horan A. C. Chemical characteristics and classification of nocardiae. Can J Microbiol. 1973 Aug;19(8):965–972. doi: 10.1139/m73-154. [DOI] [PubMed] [Google Scholar]
- ROBINSON W. G., HOOK R. H. RICININE NITRILASE. I. REACTION PRODUCT AND SUBSTRATE SPECIFICITY. J Biol Chem. 1964 Dec;239:4257–4262. [PubMed] [Google Scholar]
- Schuchmann H. P., Laidler K. J. Nitrogen compounds other than NO in automobile exhaust gas. J Air Pollut Control Assoc. 1972 Jan;22(1):52–53. doi: 10.1080/00022470.1972.10469609. [DOI] [PubMed] [Google Scholar]
- Strobel G. A. 4-amino-4-cyanobutyric acid as an intermediate in glutamate biosynthesis. J Biol Chem. 1967 Jul 25;242(14):3265–3269. [PubMed] [Google Scholar]
- Strobel G. A. The fixation of hydrocyanic acid by a psychrophilic basidiomycete. J Biol Chem. 1966 Jun 10;241(11):2618–2621. [PubMed] [Google Scholar]
- THIMANN K. V., MAHADEVAN S. NITRILASE. I. OCCURRENCE, PREPARATION, AND GENERAL PROPERTIES OF THE ENZYME. Arch Biochem Biophys. 1964 Apr;105:133–141. doi: 10.1016/0003-9861(64)90244-9. [DOI] [PubMed] [Google Scholar]
- Uematsu T., Suhadolnik R. J. In vivo and enzymatic conversion of toyocamycin to sangivamycin by Streptomyces rimosus. Arch Biochem Biophys. 1974 Jun;162(2):614–619. doi: 10.1016/0003-9861(74)90223-9. [DOI] [PubMed] [Google Scholar]
- Veldkamp H. Saprophytic coryneform bacteria. Annu Rev Microbiol. 1970;24:209–240. doi: 10.1146/annurev.mi.24.100170.001233. [DOI] [PubMed] [Google Scholar]


