Abstract
A library of “introgression lines” containing Solanum lycopersicoides chromosome segments in the genetic background of cultivated tomato (Lycopersicon esculentum) was used to study factors affecting homeologous recombination. Recombination rates were estimated in progeny of 43 heterozygous introgressions and whole-chromosome substitution lines, together representing 11 of the 12 tomato chromosomes. Recombination within homeologous segments was reduced to as little as 0–10% of expected frequencies. Relative recombination rates were positively correlated with the length of introgressed segments on the tomato map. The highest recombination (up to 40–50% of normal) was observed in long introgressions or substitution lines. Double-introgression lines containing two homeologous segments on opposite chromosome arms were synthesized to increase their combined length. Recombination was higher in the double than in the single segment lines, despite a preference for crossovers in the region of homology between segments. A greater increase in homeologous recombination was obtained by crossing the S. lycopersicoides introgression lines to L. pennellii—a phylogenetically intermediate species—or to L. esculentum lines containing single L. pennellii segments on the same chromosome. Recombination rates were highest in regions of overlap between S. lycopersicoides and L. pennellii segments. The potential application of these results to breeding with introgression lines is discussed.
AS plant breeders broaden their search for novel traits and allelic diversity, it is frequently necessary to search in the more distant wild relatives of crop plants. Introgression of genes from exotics can be restricted by pre- and postzygotic barriers that prevent or impede gene transfer. In cases where interspecific hybrids are viable but sterile, the sterility may result from genic and/or chromosomal effects (Stebbins 1958). Sterility of the latter type occurs when genomes are so diverged that homeologous chromosomes of different species fail to recombine, leading to abnormal assortment at meiosis. Meiotic recombination can also be suppressed in backcross generations, leading to linkage drag, i.e., the unintended transfer of large blocks of DNA surrounding a gene of interest.
The cultivated tomato, Lycopersicon esculentum (syn. Solanum lycopersicum), can be experimentally hybridized with each of the 9–13 species in the tomato clade (i.e., genus Lycopersicon or Solanum section Lycopersicon). The resulting F1 hybrids are relatively fertile and chromosomes undergo normal meiotic pairing and recombination processes. Comparative genetic maps constructed from interspecific tomato populations show a high degree of colinearity between genomes of all tomato species. However, recombination is typically reduced after backcrossing to cultivated tomato (Rick 1969, 1971) and somewhat lower in male than in female gametes (de Vicente and Tanksley 1991; van Ooijen et al. 1994).
Hybrids between cultivated tomato and the related nightshades S. lycopersicoides or S. sitiens (syn. S. rickii) are highly sterile, due at least in part to reduced chromosome pairing and recombination (Rick 1951; DeVerna et al. 1990). A comparative linkage map of the S. lycopersicoides/S. sitiens genome shows that it is mostly colinear with the genomes of species in the tomato clade, but with one chromosome arm (10L) involved in a paracentric inversion (Pertuze et al. 2002). Recombination in the F1 L. esculentum × S. lycopersicoides hybrid is reduced genomewide by ∼27% relative to other tomato maps (Chetelat et al. 2000). While the inversion accounts for part of this reduction—recombination is completely blocked in genotypes heterozygous for the inverted region—the genomewide effects must be due in part to excessive sequence divergence between the parental species. The L. esculentum and S. lycopersicoides genomes are readily distinguished by genomic in situ hybridization and differ in the copy number and/or locations of certain repetitive DNA elements (Ji et al. 2004). Differences were also found between them with respect to chromosome size and timing of condensation (Menzel 1962; Rick et al. 1986). Allotetraploid hybrids show preferential pairing of homologous chromosomes, with complete bivalent formation and consequently greater fertility than 2x hybrids (Menzel 1964), suggesting that the genomes of S. lycopersicoides/S. sitiens are homeologous (partially homologous) with that of cultivated tomato.
Heterozygous substitution lines, in which one tomato chromosome is substituted with one homeologous S. lycopersicoides chromosome, recombine at <50% of the rate observed in the F1 interspecific hybrid, indicating strong background effects (Ji and Chetelat 2003). Recombination is even lower (0–2% of normal) in corresponding monosomic addition lines, in which the S. lycopersicoides chromosome is present as an extra (i.e., 2n + 1), suggesting that exchange between homeologous chromosomes is antagonized by homologous associations.
We recently reported synthesis of a set of S. lycopersicoides introgression lines in the background of cultivated tomato (Canady et al. 2005). Each line contains one to several donor segments. Approximately 96% of the nightshade genome is captured in 56 such lines. In the present study, homeologous segments from different regions of the genome were compared with respect to their tendency to recombine with the tomato chromosomes. Several properties of introgressions that might affect the rate of homeologous recombination, such as segment length and position within the chromosome, were examined. Double-introgression lines of various types were synthesized to evaluate their potential for increasing recombination frequency in a region of interest. These experiments tested the importance of two principal variables affecting homeologous recombination in interspecific hybrids: the preference for homologous exchange within the same chromosome and the degree of sequence divergence between introgressed segments and the recipient genome.
MATERIALS AND METHODS
Plant material:
A group of 43 S. lycopersicoides introgression lines and substitution lines were used in this study (Table 1). The parental genotypes and breeding strategy used for selecting the lines were described previously (Ji and Chetelat 2003; Canady et al. 2005). Briefly, both types of prebreds were derived from S. lycopersicoides accession LA2951 by backcrossing to L. esculentum cv. VF36. The lines used in the current study cover most of the genome. Several genomic regions are excluded from this analysis, including regions of chromosomes 2, 3, and 4 for which no introgressed segments were recovered, and chromosomes 5, 9, and 11 for which heterozygous lines were not available.
TABLE 1.
Frequency of parental and recombinant genotypes in progeny of S. lycopersicoides introgression lines
| No. of plantsd
|
Recombination rate (cM)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Line | Flanking markersa | Positionb | Gener.c | NR | SCOs | DCOs | Obs. | Exp.e | % Obs./Exp. |
| 1 | LA4231 | TG301–TG83 | I/Pe | F2 | 34 | 5 | 0 | 6.9 | 72.3 | 9.5 |
| 1 | LA4295 | TG51–TG465 | I/Pe | F2 | 68 | 3 | 0 | 2.1 | 67.6 | 3.1 |
| 1 | LA4294 | TG192–TG71 | I/Pe | F2 | 75 | 0 | 0 | 0 | 26.8 | 0 |
| 1 | LA4296 | TG192–TG71 | I/Pe | F2 | 89 | 1 | 0 | 0.57 | 26.8 | 2.1 |
| 1 | LS15-2AC | TG192–TG465 | I/Pe | F2 | 42 | 3 | 0 | 3.5 | 58.5 | 6.0 |
| 1 | LA4232 | TG343–TG83 | I/Pa | F2 | 101 | 0 | 0 | 0 | 8.7 | 0 |
| 1 | LA4233 | TG83–TG17 | I/Pa | F2 | 52 | 2 | 0 | 1.9 | 33.2 | 5.7 |
| 1 | LA4234 | TG333–TG27 | T/Pa | F2 | 26 | 6 | 0 | 10.9 | 31.9 | 34.2 |
| 1 | LA4235 | TG267A–TG27 | T/Pa | F2 | 27 | 1 | 0 | 1.8 | 11.5 | 15.7 |
| 1 | LA4235 | TG267A–TG27 | T/Pa | BC ♀ | 82 | 0 | 0 | 0 | 11.5 | 0 |
| 1 | LA4235 | TG267A–TG27 | T/Pa | BC ♂ | 47 | 0 | 0 | 0 | 11.5 | 0 |
| 2 | LA4239 | TG48–TG507 | T/Pa | F2 | 112 | 5 | 0 | 2.2 | 42.9 | 5.1 |
| 2 | LA4237 | TG554–TG308 | I/Pa | F2 | 94 | 0 | 0 | 0 | 16.9 | 0 |
| 3 | LA4241 | TG479–TG288 | T/Pe | F2 | 49 | 2 | 0 | 2.0 | 56.4 | 3.6 |
| 3 | LA4242 | TG42–TG244 | T/Pa | F2 | 32 | 0 | 0 | 0 | 50.1 | 0 |
| 4 | LA4244 | TG49–TG146 | T/Pa | F2 | 24 | 0 | 0 | 0 | 25.3 | 0 |
| 4 | LA4244 | TG49–TG146 | T/Pa | F2 | 36 | 0 | 0 | 0 | 25.3 | 0 |
| 4 | LA4245 | Tpi-2–Adh-1 | T/Pe | F2 | 110 | 5 | 0 | 2.2 | 24.9 | 8.9 |
| 4 | LA4246 | CT50–TG464 | T/Pa | F2 | 72 | 0 | 0 | 0 | 24.3 | 0 |
| 6 | LA4300 | TG297–TG153 | T/Pe | F2 | 25 | 0 | 0 | 0 | 29.3 | 0 |
| 6 | LA4253 | TG297–Adh-2 | T/Pe | F2 | 155 | 0 | 0 | 0 | 34.8 | 0 |
| 6 | LA4254 | TG153–TG292 | I/Pa | F2 | 141 | 3 | 0 | 1.1 | 30.8 | 3.4 |
| 6 | LA4255 | TG292–CT206 | I/Pa | F2 | 21 | 0 | 0 | 0 | 22.8 | 0 |
| 6 | LA3881 | TG548–TG220 | T/Pa | F2 | 19 | 0 | 0 | 0 | 27.6 | 0 |
| 7 | LA4261 | TG252–TG342 | T/Pe | F2 | 64 | 6 | 0 | 4.4 | 29.3 | 14.9 |
| 7 | SL-7sub | TG199–TG342 | T/Pe | F2 | 41 | 9 | 0 | 9.3 | 76.2 | 12.2 |
| 7 | LA4259 | TG499–TG128 | T/Pa | F2 | 96 | 6 | 0 | 3.0 | 42.0 | 7.1 |
| 7 | LA4258 | TG499–TG216 | T/Pa | F2 | 110 | 1 | 0 | 0.46 | 29.5 | 1.6 |
| 7 | LA4315 | TG499–TG342 | S | BC ♀ | 57 | 25 | 2 (2) | 33.8 | 92.1 | 36.7 |
| 7 | LA4315 | TG499–TG342 | S | F2 | 39 | 50 | 10 (0) | 37.9 | 92.1 | 41.2 |
| 8 | LA4305 | TG176–TG41 | T/Pe | F2 | 41 | 0 | 0 | 0 | 24.0 | 0 |
| 8 | LS9-26 | TG176–TG510 | T/Pe | F2 | 48 | 3 | 0 | 3.0 | 61.4 | 4.9 |
| 8 | LS4-13 | TG510–TG294 | T/Pa | F2 | 47 | 0 | 0 | 0 | 30.9 | 0 |
| 8 | LA4265 | TG624–TG510 | I/Pa | F2 | 59 | 1 | 0 | 0.86 | 24.9 | 3.5 |
| 8 | LA4307 | TG176–TG294 | S | BC ♀ | 39 | 16 | 0 | 29.1 | 94.7 | 30.7 |
| 8 | LA4307 | TG176–TG294 | S | F2 | 74 | 43 | 10 (5) | 26.2 | 94.7 | 27.7 |
| 9 | LA4270 | TG18–TG186 | T/Pe | F2 | 102 | 11 | 3 (2) | 7.4 | 53.6 | 13.9 |
| 10 | LA4274 | TG303–TG43 | I/Pa | F2 | 43 | 0 | 0 | 0 | 20.7 | 0 |
| 10 | LA4276 | TG408–CD32B | T/Pa | F2 | 168 | 0 | 0 | 0 | 52.8 | 0 |
| 11 | LA4277 | TG557–TG523 | T/Pa | F2 | 107 | 3 | 0 | 1.4 | 26.5 | 5.3 |
| 12 | LA4313 | TG180–TG68 | T/Pa | F2 | 29 | 0 | 0 | 0 | 13.8 | 0 |
| 12 | LA4283 | TG111–CT156 | I/Pa | F2 | 30 | 0 | 0 | 0 | 25.0 | 0 |
| 12 | LA4282 | TG180–TG111 | T/Pe | F2 | 60 | 15 | 1 (0) | 12.1 | 47.1 | 25.7 |
Chr., chromosome; Gener., generation; Obs., observed; Exp., expected.
Represent end markers on each introgressed segment.
I, interstitial; T, terminal; Pa, paracentric; Pe, pericentric; S, substituted chromosome.
BC ♀, backcross, heterozygote used as female parent.
NR, nonrecombinant; SCO, single-crossover genotypes; DCO, double-crossover genotypes (in parentheses, the number of DCOs that must have occurred in the same gamete).
Expected recombination rate for the same marker interval from the reference map of tomato (Tanksley et al. 1992).
Double-introgression lines were constructed by crossing selected lines with single segments. Heterozygosity for both segments was confirmed with RFLP markers. Some S. lycopersicoides introgression lines were also crossed to L. pennellii (syn. S. pennellii) accession LA0716, a self-compatible and completely homozygous strain of this wild species. Others were crossed to stocks containing single introgressed segments from L. pennellii in the background of L. esculentum cv. M82, developed by Eshed and Zamir (1995) and Liu and Zamir (1999). Seeds of all plant materials used in this study were obtained from the C. M. Rick Tomato Genetics Resource Center at the University of California (Davis, CA).
Recombination frequency was measured by genotyping F2 (and in a few instances, backcross, BC) progeny of heterozygous introgression or substitution lines. F2 seeds were obtained by allowing heterozygotes to self-pollinate and backcross seeds by crossing heterozygotes to “VF36,” the background genotype for these lines. Seeds of segregating F2 and BC populations were treated for 30 min with 50% household bleach (equivalent to 2.75% sodium hypochlorite), rinsed under running tap water for several minutes, plated on germination paper in plastic boxes, and incubated at 25° under a 12-hr photoperiod. Seedlings with fully expanded cotyledons were transplanted to artificial soil mix in the greenhouse and were genotyped at the three-to-four true leaf stage.
Marker analysis:
DNA marker analysis was used to measure recombination within introgressed segments. For each introgressed segment, a minimum of two markers were used, one from each end. Markers, primarily RFLPs, were chosen to mark the ends of each segment, according to their locations on the high-density molecular linkage map of tomato (Tanksley et al. 1992). For substitutions and longer introgressed segments, more than two markers were used to detect multiple crossovers. DNA extractions and RFLP analysis were performed as previously described (Canady et al. 2005).
Statistical analysis:
In cases where marker data were available from two or more independent progeny tests of the same heterozygous introgression line, a chi-square test for independence was used to decide if data from the separate tests could be pooled. The maximum-likelihood method was used to estimate recombination rates using either LINKAGE-1 (Suiter et al. 1983) or Mapmaker v3.0 (Lander et al. 1987). The threshold parameters used for detecting linkage were a chi-square test with P < 0.05 for LINKAGE-1 or a LOD ≥ 3.0 and recombination fraction ≤30% for Mapmaker. Genetic distances in centimorgans were calculated from recombination fraction estimates using the Kosambi mapping function. Student's t-test was used to make pairwise comparisons of mean recombination values in peri- vs. paracentric and terminal vs. interstitial S. lycopersicoides segments.
Expected values for recombination rates were obtained from the genetic distances in the corresponding marker intervals on the RFLP map of tomato. Derived from an interspecific cross of L. esculentum × L. pennellii, the reference map is not, strictly speaking, a control for purely homologous recombination rates. However, due to the lack of marker polymorphism within the cultivated gene pool, the interspecific map provides the best available estimates of recombination rates useful for comparisons across different mapping populations. Furthermore, L. esculentum × L. pennellii hybrids are fertile, and their chromosomes pair normally during meiosis (Khush and Rick 1963), indicating they are functionally homologous at this level. Recombination frequencies in the double introgressions were compared to the single-segment controls using chi-square contingency tests on the numbers of parental vs. recombinant progeny. To compare F2 with BC populations, the chi-square test was carried out on the numbers of parental vs. recombinant gametes rather than on individual plants.
RESULTS
Recombination in single-introgression lines:
Estimates of homeologous recombination frequencies were obtained from F2 and BC progeny of 43 introgression and substitution lines, representing 11 of 12 S. lycopersicoides chromosomes. The total genetic length of the marker intervals for which these lines were heterozygous (i.e., in which recombination could be measured) constituted ∼68% of the map units in the tomato genome. Most of the lines were nearly isogenic, i.e., contained a single introgressed segment, while a small number contained one to several extra segments on other chromosomes (Canady et al. 2005). Certain introgressions on chromosomes 3, 9, and 12 were homozygous for S. lycopersicoides markers at one end of the introgressed region or at the other end of the chromosome (Figure 1).
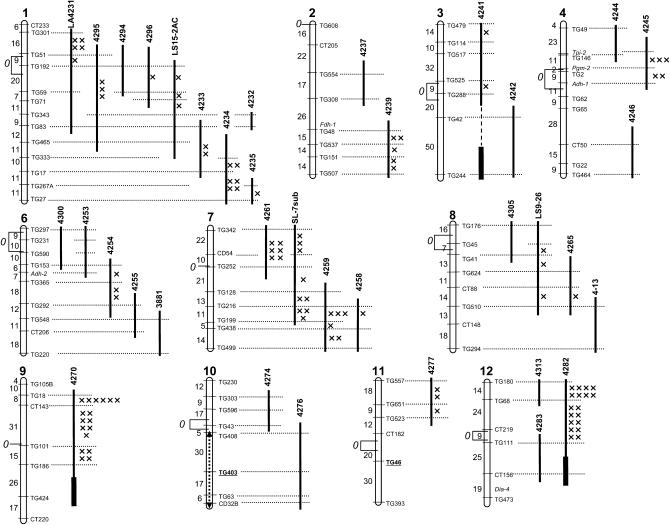
Figure 1.—
Genetic map of S. lycopersicoides introgression lines. The introgressed segments in each line are indicated by solid bars to the right of the chromosome maps. Thicker lines indicate regions homozygous for S. lycopersicoides markers. Numbers above the line are the accession identifiers (all “LA” numbers unless otherwise indicated). Dashed lines connecting marker loci to the introgressed segments indicate which markers were evaluated in each line. The locations of recombination events detected in progeny are indicated by 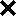 's. (An absence of
's. (An absence of 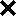 's in a given interval indicates no crossovers were detected). Recombination data are summarized in Table 1. The distances between markers are from Tanksley et al. (1992), and the positions of centromeres (0) are from Pillen et al. (1996). The location of a paracentric inversion on chromosome 10 that distinguishes cultivated tomato from S. lycopersicoides is shown by a dashed line with double arrowheads (from Pertuze et al. 2002).
's in a given interval indicates no crossovers were detected). Recombination data are summarized in Table 1. The distances between markers are from Tanksley et al. (1992), and the positions of centromeres (0) are from Pillen et al. (1996). The location of a paracentric inversion on chromosome 10 that distinguishes cultivated tomato from S. lycopersicoides is shown by a dashed line with double arrowheads (from Pertuze et al. 2002).
Recombination between the L. esculentum chromosome and the introgressed S. lycopersicoides segments was greatly reduced, in most cases to only 0–10% of the expected values (Table 1, Figure 2). This reduction was genomewide, occurring on all tested chromosomes. Double-crossover genotypes were extremely rare, indicating a strong crossover interference in most cases (Table 1). Crossovers were approximately randomly distributed within the introgressed segments (Figure 1). Intervals between more distant markers (on the tomato RFLP map) generally had more recombination events, as expected. However, for introgressed segments in which more than one marker interval was tested, there seemed no preference for crossovers in distal vs. proximal regions (Figure 1). Despite the overall lower level of homeologous recombination, crossover events were recorded in most regions of the genome, with a few exceptions, such as chromosome 10 and the short arm of chromosome 6 (Figure 1).
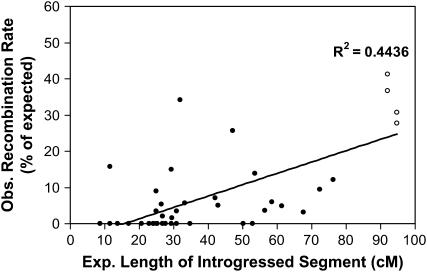
Figure 2.—
Correlation between observed frequencies of homeologous recombination in S. lycopersicoides introgression lines (expressed as a percentage of the expected value) and the genetic length of introgressed segments on the tomato map. Observed recombination rates represent the combined map units of all marker intervals in each segment. The expected genetic lengths are the distances between corresponding markers on the RFLP map of tomato (Tanksley et al. 1992). Included in the correlation are four data points (open circles) representing recombination frequencies in substitution lines containing S. lycopersicoides chromosome 7 or 8 (SL-7, SL-8). Note that data on LA4276 are not included because this line carries an inverted segment that does not recombine.
In general, lines with “longer” segments (i.e., greater map units on the RFLP map) recombined more frequently than those with shorter segments, as might be expected (Table 1). Significantly, this trend was true even when recombination frequencies were normalized for expected genetic length on the basis of the RFLP map. In fact, the highest “relative” recombination frequencies were observed in the substitution lines, which were heterozygous for an intact S. lycopersicoides chromosome. A positive correlation was observed between the expected genetic lengths of introgressed segments and the observed relative rates of homeologous recombination in the introgressed region (Figure 2). This correlation was also observed within individual chromosomes. For instance, on chromosomes 7 and 8, the highest recombination frequencies were observed in the whole-chromosome substitutions. Introgression lines with relatively long homeologous segments (>50% of the length of the chromosome) were intermediate, and those with short segments recombined at the lowest rates (Table 1, Figure 2).
A few exceptions to these trends were noted. Line LA4234, which contained a fairly short S. lycopersicoides introgression (31.9 cM on the tomato map) on chromosome 1, recombined at a rate higher than average (34.2% of expected) (Table 1). In contrast, no recombination was detected in line LA4242, which contained a longer introgressed segment (>50 cM) on chromosome 3. As expected, no recombination was observed in LA4276, which contained a segment spanning the whole long arm of chromosome 10; recombination in this region is prevented by a paracentric inversion in S. lycopersicoides relative to L. esculentum and thus is irrelevant to the relationship between length and recombination frequency. This line was therefore excluded from further analysis.
Average recombination estimates were calculated for introgressions according to the relative positions of S. lycopersicoides and L. esculentum segments within the chromosomes (Table 2). A chromosome segment was considered “terminal” if it included one end of the chromosome or “interstitial” if it did not. Alien segments that spanned the centromere (“pericentric”) were distinguished from those that were limited to one arm (“paracentric”). On average, terminal segments tended to recombine at a higher rate than interstitial ones; however, the difference was not significant (t = 1.42, P < 0.1). Although pericentric segments showed a higher recombination rate than paracentric ones, the former were also longer on average. Thus any difference in recombination frequency between these two categories is confounded by segment length in addition to position effects. As mentioned previously, the substituted chromosomes recombined at higher rates than any of the introgressed segments.
TABLE 2.
Average recombination frequencies within S. lycopersicoides introgressed segments according to their positions within chromosomes relative to telomeres and centromeres
| Position relative to
|
Obs. recombination
|
||||
|---|---|---|---|---|---|
| Telomerea | Centromereb | No. of linesc | Exp. recombination (cM)d | cM | % of expected |
| Interstitial | Paracentric | 8 | 22.9 | 0.48 | 1.6 |
| Pericentric | 5 | 50.4 | 2.6 | 4.1 | |
| Either | 13 | 33.5 | 1.3 | 2.6 | |
| Terminal | Paracentric | 15 | 27.0 | 1.3 | 4.6 |
| Pericentric | 10 | 43.7 | 4.0 | 8.4 | |
| Either | 25 | 33.7 | 2.4 | 6.1 | |
| Substitution | Pericentric | 4 | 93.4 | 31.8 | 34.1 |
| All | — | 42 | 39.3 | 4.9 | 7.7 |
Exp., expected; Obs., observed.
Terminal, includes one end of the chromosome; interstitial, does not include a chromosome end; Substitution, an intact alien chromosome.
Pericentric, includes the centromeric region; paracentric, does not include the centromere.
Number of lines in each category.
Expected recombination rates are estimated as the length of introgressed segments on the reference map (Tanksley et al. 1992).
Recombination in “target/driver” introgression lines:
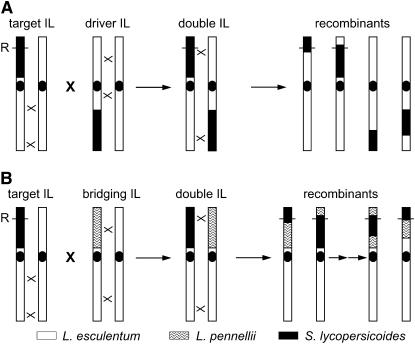
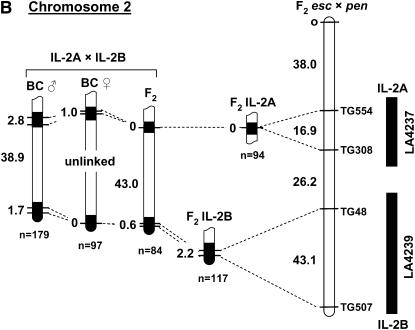
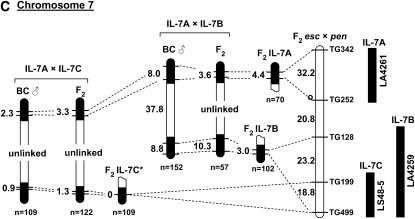
The positive correlation we observed between recombination frequency and the length of S. lycopersicoides segments suggested a possible strategy to increase the probability of recovering recombinants. By combining two introgressed segments from different regions of the same chromosome, the total length of homeology could be increased (Figure 3A). We refer to such a double introgression as a “target/driver” genotype, wherein the target segment would contain a gene of interest (e.g., a disease resistance locus), around which recombinants are desired. The driver segment would contain a different S. lycopersicoides introgression, preferably on the opposite chromosome arm. In the doubly heterozygous F1 hybrid, these two segments would initially be oriented in repulsion phase. Recombinants would be selected in F2 progeny by marker analysis. In theory, two overlapping terminal segments combined in this fashion would be similar or equivalent to a substitution line, except for their linkage phase. To test this hypothesis, we made four pairs of double-introgression lines combining S. lycopersicoides segments on chromosomes 1, 2, and 7 (Figure 4). These represented several different configurations of the two segments: two interstitial segments on the same arm (chromosome 1), one interstitial and one terminal on the same arm (chromosome 2), and two terminal segments on opposite arms, with either a small or a large region of homology between them (chromosome 7). The controls for the target/driver genotypes were the corresponding single-segment introgression lines.
Figure 3.—
Diagram illustrating the construction of double S. lycopersicoides introgression lines, and their possible use for increasing recombination frequency in the progeny. The “target” introgression line contains a homeologous segment with a gene of interest, such as a hypothetical disease resistance factor (R). (A) The “driver” line contains a homeologous segment on the opposite arm of the same chromosome. (B) The “bridging” introgression is a line containing a donor segment from L. pennellii. Each double-introgression line is heterozygous for two alien segments, initially in repulsion linkage phase, on the same chromosome. The locations of crossover events, predicted to occur preferentially in homologous stretches, are indicated with an  . Representative recombinant chromosomes that are obtainable in the progeny are shown.
. Representative recombinant chromosomes that are obtainable in the progeny are shown.
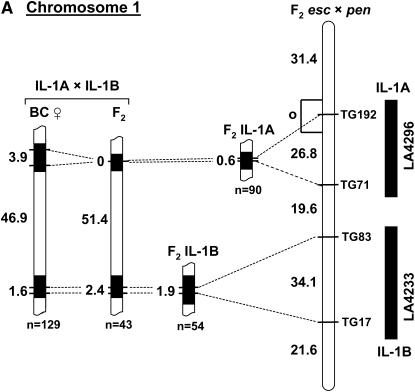
Figure 4.—
Recombination in “target/driver” double-introgression lines for chromosomes 1 (A), 2 (B), and 7 (C). For each chromosome, the location of the two introgressed segments is shown to the right of the reference map, based on recombination in F2 L. esculentum × L. pennellii (F2 esc × pen). The total map units in each interval are shown to the left of the reference map. Recombination in single introgressed segments served as controls for the corresponding double-introgression lines. Linkage estimates were obtained both from F2 and from backcross (BC) progeny. The rate of recombination was measured both within the introgressed segments (solid regions, indicating homeology) and in the interval between them (open segments, indicating homology). The total number of individuals genotyped in each progeny array is indicated by n. The asterisk on the IL-7C map is to indicate that the control data for this marker interval were from a line with a slightly longer segment, extending beyond TG199 to marker TG216 (not shown on the map).
The chromosome 1 and 2 target/driver combinations showed some evidence of increased homeologous recombination relative to the single-segment controls (Figure 4, A and B). For each chromosome, one of the two S. lycopersicoides segments (1A and 2A, respectively) recombined at higher rates than the controls, but only in the BC populations, not the F2's. For segment 1A, an increase of >6-fold was observed (χ2 = 4.37, P < 0.05), and for segment 2A, recombination increased from 0 to 2.8 cM (χ2 = 7.84, P < 0.01). Interestingly, it was the more proximal segment on both chromosomes that showed the increase, while the distal segments showed little or no change. Both double-introgression lines for chromosome 7 showed elevated recombination frequencies for the segments on the long arm in each pair, 7B and 7C (Figure 4C). Recombination within the 7B segment increased by ∼3-fold in the target/driver genotypes relative to the single-segment control (χ2 = 5.6, P < 0.025 for the F2 and χ2 = 5.4, P < 0.025 for the BC population). Recombination rate in the 7C segment increased from 0 in the control to up to 1.3 cM in the F2 combination stock (χ2 = 4.2, P < 0.05). The frequency of recombination within the longer segment 7B was ∼10-fold higher than that observed in the shorter segment 7C. This is consistent with our observation of lower recombination in short than in long introgressed segments (Figure 2).
A more pronounced increase in recombination was observed in the interval between the introgressed segments, i.e., in the intercalary stretch of homology (Figure 4). Recombination frequencies in these “gaps” could be estimated because each target/driver combination (e.g., 1A + 1B) was heterozygous for markers flanking the homozygous interval. In contrast, the lines with a single homeologous segment provided no information on recombination in these regions, because they were heterozygous for markers on one side only. We therefore used genetic distances between the same markers on the reference map of tomato (from F2 L. esculentum × L. pennellii) as “controls.” In each case, recombination rate in these homozygous regions between the paired segments was increased relative to the reference map. For example, on chromosome 1, the length of the TG71–TG83 interval increased from 19.6 cM (from the reference map) to ∼50 cM in the double-segment lines. In half of the mapping populations, the increase in recombination within these gaps was so great that linkage between the flanking markers could not be detected. These observations suggest that reduced recombination within regions of homeology (i.e., S. lycopersicoides segments) is compensated by an increase in recombination within adjacent—in this case intercalary—regions of homology.
This effect appears to be due in part to selection toward some recombinants genotypes. Significant segregation distortion was observed in many of the target/driver genotypes (Table 3). In every case, the recombinant classes showed an excess of genotypes that had lost one segment through recombination (i.e., +–+) and a deficiency of genotypes that had gained a segment (i.e., A–B in coupling phase). The most pronounced distortion of this type was on chromosome 1, where the BC population produced 47 +–+ vs. 0 A–B recombinants. On chromosome 7, the ratio of +–+ to A–B recombinants was as high as 4:1.
TABLE 3.
Segregation in progeny of double-introgression lines containing two S. lycopersicoides segments on the same chromosome pair, oriented in repulsion phase, and corresponding single-segment control lines
| Genotypic classesb
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Cross (♀ × ♂)a | Line | A +/A + | A +/+ B or + +/A B | + B/+ B | + +/+ + | + +/A + | + +/+ B | A B/A + | A B/+ B | A B/A B | nc | Exp. ratio | χ2d |
| 1 | Control F2 (1A/+) × self | LA4296 | 20 | 16 | 53 | 89 | 1:2:1 | 3.60 (NS) | ||||||
| 1 | Control F2 (1B/+) × self | LA4233 | 1 | 6 | 45 | 52 | 1:2:1 | 28.7*** | ||||||
| 1 | F2 (1A +/+ 1B) × self | 3 | 11 | 3 | 1 | 10 | 5 | 2 | 8 | 0 | 43 | 1:2:1 | 1.47 (NS) | |
| 1 | BC (1A +/+ 1B) × + + | 0 | 47 | 39 | 42 | 128 | 1:1 | 0.049 (NS) | ||||||
| 2 | Control F2 (2A/+) × self | LA4237 | 13 | 32 | 49 | 94 | 1:2:1 | 7.85*** | ||||||
| 2 | Control F2 (2B/+) × self | LA4239 | 16 | 44 | 52 | 112 | 1:2:1 | 14.6*** | ||||||
| 2 | F2 (2A +/+ 2B) × self | 2 | 25 | 9 | 1 | 15 | 13 | 5 | 11 | 0 | 81 | 1:2:1 | 6.52* | |
| 2 | BC (2A +/+ 2B) × + + | 17 | 23 | 20 | 36 | 96 | 1:1 | 4.02* | ||||||
| 2 | BC + + × (2A +/+ 2B) | 24 | 33 | 51 | 67 | 175 | 1:1 | 1.91 (NS) | ||||||
| 7 | Control F2 (7A/+) × self | LA4261 | 4 | 43 | 17 | 64 | 1:2:1 | 61.6*** | ||||||
| 7 | Control F2 (7B/+) × self | LA4259 | 20 | 24 | 52 | 100 | 1:2:1 | 1.00 (NS) | ||||||
| 7 | Control F2 (7C/+) × self | LA4258 | 37 | 31 | 41 | 109 | 1:2:1 | 7.35*** | ||||||
| 7 | F2 (7A +/+ 7B) × self | 0 | 8 | 18 | 3 | 4 | 15 | 0 | 5 | 0 | 53 | 1:2:1 | 28.8*** | |
| 7 | F2 (7A +/+ 7C) × self | 1 | 19 | 16 | 10 | 6 | 44 | 2 | 17 | 3 | 118 | 1:2:1 | 12.6** | |
| 7 | BC + + × (7A +/+ 7B) | 15 | 30 | 14 | 82 | 141 | 1:1 | 46.8*** | ||||||
| 7 | BC + + × (7A +/+ 7C) | 9 | 29 | 9 | 40 | 87 | 1:1 | 18.4*** | ||||||
The introgressed segments in each line are shown on the maps in Figure 4, where A denotes the segment on the short arm and B the segment on the long arm of the same chromosome. The 7C segment on chromosome 7 is considered the B segment. The wild-type or L. esculentum alleles at the corresponding marker loci are indicated by a +. Note that the control, single-segment populations were grown separately.
Segregation data are the number of plants in each genotypic class; underlined values indicate genotypes with crossovers between the A and B segments.
n is the sample size, excluding individuals with recombination within the A or B segments.
Chi-square values test for goodness-of-fit to expected Mendelian ratios and are based on data in the parental (nonrecombinant) classes only. Significance levels are: NS, not significant; *P < 0.05, **P < 0.01, and ***P < 0.001.
Recombination between S. lycopersicoides and L. pennellii:
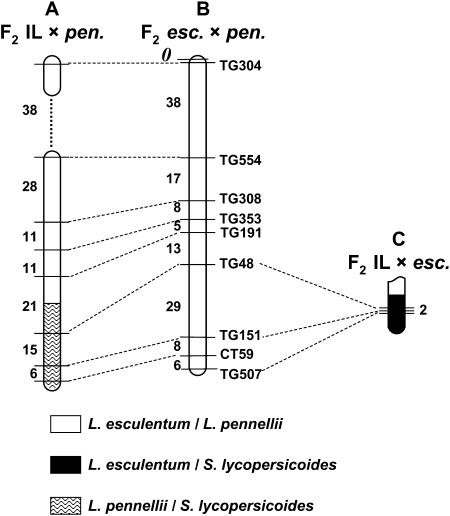
The reduced recombination we observed between tomato chromosomes and the homeologous S. lycopersicoides segments likely results from excessive sequence divergence between the two genomes. We therefore hypothesized that S. lycopersicoides introgressed segments might recombine more readily with DNA from a species of closer sequence similarity. To test this concept, we crossed an introgression line containing a S. lycopersicoides segment on chromosome 2 with L. pennellii, a wild species that is phylogenetically intermediate between tomato and the nightshade (Figure 5). In the genetic background of L. esculentum, this introgressed region recombined at only 4.7% of the expected value. However, in F2 progeny of the introgression line × L. pennellii hybrid, the total genetic length of the S. lycopersicoides segment was increased to 21 cM, an ∼10-fold increase, but still less than the homologous recombination rate inferred from the F2 L. esculentum × L. pennellii map. This result suggests that S. lycopersicoides chromosome segments recombine more readily with L. pennellii than with L. esculentum DNA. Interestingly, recombination along the rest of the chromosome, i.e., between L. esculentum and L. pennellii DNA, was slightly increased compared to the reference map, particularly in the intervals flanking the S. lycopersicoides segment. This provides further evidence that reduced recombination within a region of homeology is accompanied by increased crossing over in the adjacent homologous regions.
Figure 5.—
Genetic maps of recombination frequencies within S. lycopersicoides introgression lines for chromosome 2 in different genetic backgrounds. (A) Recombination in F2 progeny of introgression line LS38-11 crossed to L. pennellii. (B) Genetic distances between the same markers taken from the RFLP map of tomato (Tanksley et al. 1992). (C) Recombination in F2 progeny of LA4239 in the background of L. esculentum (data from Table 1). Chromosomes are shaded to indicate that the genetic distances are in centimorgans, based on the Kosambi mapping function. Map A consisted of two linkage groups at the threshold of LOD ≥ 3.0.
However, the increased recombination between the S. lycopersicoides segment and the L. pennellii chromosome could result from genetic background effects. For example, there might be genes affecting pairing or recombination on other L. pennellii chromosomes (i.e., other than chromosome 2, in this case). Alternatively, there could be a genomewide enhancement of recombination due to the interspecific nature of the hybrids with L. pennellii. Finally, from a breeding standpoint, crossing prebreds already in a cultivated tomato background to pure L. pennellii would be an inefficient method to increase recombination since extensive backcrossing and selection would be needed to eliminate the genome of the latter species.
To address these issues, we took advantage of a similar set of introgression lines containing L. pennellii segments in the background of cultivated tomato. We crossed lines containing a segment from S. lycopersicoides to those containing one from L. pennellii on the same chromosome (Figure 3B). We refer to the L. pennellii introgressions in this context as “bridging introgressions” to reflect their phylogenetic connection to both the nightshade and the cultivated tomato. If the rate of homeologous recombination is primarily limited by the degree of sequence homology, then the bridging genotypes should increase recombination relative to the single-segment controls.
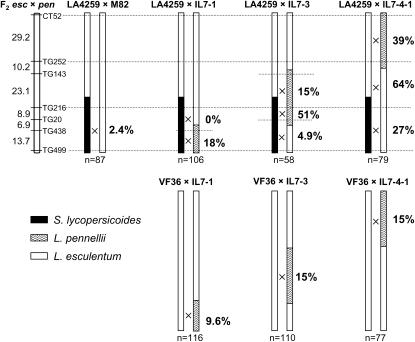
To test this hypothesis, we measured recombination within a segment from S. lycopersicoides chromosome 7 (LA4259), either alone or in combination with three different L. pennellii segments on the same chromosome (Figure 6). To eliminate the effect of differences in the genetic background between the two sets of introgressions—“M82” was used for the L. pennellii and VF36 for the S. lycopersicoides derivatives—each single introgression was crossed to the parent of the other set so that all recombination measurements were made in a constant genetic background, equivalent to VF36 × M82. Recombination rates were normalized to expected frequencies from the reference map of tomato to compare relative recombination rates across different marker intervals.
Figure 6.—
Recombination in “bridging” introgression lines containing introgressed segments from S. lycopersicoides and L. pennellii on chromosome 7. Dotted lines indicate the marker loci used to measure recombination within each segment and their positions on the RFLP map (Tanksley et al. 1992). Recombination frequencies are expressed as the percentage of the expected values for the same marker intervals. An 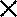 indicates the region to which each recombination value applies. The positions of the L. pennellii segments are from Liu and Zamir (1999). The controls are the lines containing a single introgressed segment, either from S. lycopersicoides (LA4259) or from L. pennellii. The single-segment L. pennellii controls were genotyped with the same markers as the corresponding segments in the double-introgression stocks. Recombination estimates are based on F2 populations (of size n) corresponding to the crosses indicated above the chromosomes. Single-introgression lines were crossed to L. esculentum cv. M82 or VF36 so that all recombination tests were carried out in a constant genetic background, equivalent to F1 VF36 × M82.
indicates the region to which each recombination value applies. The positions of the L. pennellii segments are from Liu and Zamir (1999). The controls are the lines containing a single introgressed segment, either from S. lycopersicoides (LA4259) or from L. pennellii. The single-segment L. pennellii controls were genotyped with the same markers as the corresponding segments in the double-introgression stocks. Recombination estimates are based on F2 populations (of size n) corresponding to the crosses indicated above the chromosomes. Single-introgression lines were crossed to L. esculentum cv. M82 or VF36 so that all recombination tests were carried out in a constant genetic background, equivalent to F1 VF36 × M82.
Alone, the S. lycopersicoides segment in LA4259 recombined at only 2.4% of the expected rate. Recombination within the single-segment L. pennellii line was higher—10–15% of normal—consistent with the closer homology between L. pennellii and cultivated tomato. In the LA4259 × IL7-1 hybrid, recombination within the homeologous S. lycopersicoides segment increased by over sixfold, to 18% of normal, but only in the region of overlap with L. pennellii DNA; no recombination events were recovered in the nonoverlapping region. In the LA4259 × IL7-3 cross, recombination was increased to 51% of normal in the overlapping region between L. pennellii and S. lycopersicoides segments; again, little or no change in the recombination rate was detected in the flanking regions. The last combination tested, LA4259 × IL7-4-1, consisted of two nonoverlapping segments on opposite arms of chromosome 7. Recombination was increased in both the S. lycopersicoides and the L. pennellii segments—to 27% of normal in the former, 39% in the latter—relative to their single-segment controls. These results suggest that in addition to the role of sequence homology, the bridging introgression lines may elevate homeologous recombination by a mechanism similar to that of the target/driver genotypes.
DISCUSSION
Genetic improvement of crop plants has depended to a large extent on disease resistances and other novel traits originating in wild relatives. Various types of prebred stocks have been used for this purpose in different crop plants. Sets of introgression lines representing whole genomes of related wild species, although time consuming to synthesize, provide permanent resources for mapping projects and superior starting material for breeding programs (Zamir 2001). However, a potential disadvantage is that recombination between alien (homeologous) segments and the recipient genome can be reduced.
In tomato, recombination within segments derived from L. pennellii and L. hirsutum is typically ∼15–30% of normal levels (Alpert and Tanksley 1996; van Wordragen et al. 1996; Monforte and Tanksley 2000). The recombination rates we observed for L. pennellii chromosome 7 introgression lines also fell into this range. Previous studies using morphological markers have shown that recombination between L. esculentum and L. pennellii chromosomes drops off during successive backcross generations (Rick 1969, 1971). Thus one of the advantages of introgression lines—their more uniform genetic background—is a potential obstacle when searching for recombinants. This problem is exacerbated in genomic regions subject to low recombination rates. The pericentromeric regions of each tomato chromosome have lower than average recombination rates per unit of physical distance (Tanksley et al. 1992; Sherman and Stack 1995). As a result, genes located near the centromeres, such as the nematode resistance gene Mi (Kaloshian et al. 1998), can be difficult to isolate by positional cloning. In contrast, genes located in recombination hotspots are more amenable to map-based cloning. For example, recombination in the region of the soluble-solids QTL Brix9-2-5 was so high that the effects of the L. pennellii allele could be mapped to a single amino acid (Fridman et al. 2004). From a breeding standpoint, linkage drag can make it difficult to combine tightly linked resistance genes from different sources (i.e., originally in trans configuration) into a single inbred parent (i.e., in cis orientation).
Recombination in the introgression lines is limited by sequence divergence:
In this study, we observed a genomewide reduction in recombination frequencies within introgressed S. lycopersicoides segments, often to as low as 0–10% of normal levels. These values are generally lower than previously reported for similar L. pennellii or L. hirsutum derivatives, consistent with molecular systematic studies that indicate that S. lycopersicoides is more distantly related to cultivated tomato (Peralta and Spooner 2001; Spooner et al. 2005). Evidence from other model systems, including bacteria (Shen and Huang 1986), yeast (Datta et al. 1996), and Arabidopsis (Li et al. 2006), among others, has clearly demonstrated that recombination is strongly dependent on the degree of sequence identity and can be inhibited by as little as a single-nucleotide mismatch. In a sample of coding and noncoding sequences, the nucleotide divergence between L. esculentum and L. pennellii varied from 0 to 6.3% (Nesbitt and Tanksley 2002). Our analysis of published waxy gene sequences (from Peralta and Spooner 2001) found ∼6% divergence between L. esculentum and S. lycopersicoides, compared to 2% between L. pennellii and L. esculentum (data not shown). These data provide further evidence that recombination between tomato chromosomes and orthologous segments introgressed from wild relatives is strongly influenced by their degree of sequence homology.
The above considerations do not take into account potential disruptions in chromosomal synteny that might differentiate these species and could potentially suppress recombination independent of nucleotide divergence. In hexaploid wheat, for example, the homeologous A, B, and D genomes differ by gene duplications and deletions (Akhunov et al. 2003); the B genome showed the greatest loss of synteny, which may explain why this genome undergoes less pairing with homeologous chromosomes of the A and D genomes (in the absence of Ph1) than A and D homeologues with each other. Larger-scale rearrangements, such as chromosomal inversions and translocations, would be expected to strongly suppress recombination. Comparative linkage maps have so far revealed few rearrangements among species within the Lycopersicon clade. One exception is a paracentric inversion involving part of the short arm of chromosome 7 in L. pennellii relative to L. esculentum (van der Knaap et al. 2004). This inverted region is located within the introgressed L. pennellii segment in the IL 7-4-1 line, which was included in the present study. This line recombined at ∼15% of normal, similar to the other L. pennellii derivatives. However, our reference for the expected recombination frequencies was the high-density RFLP map of tomato (from Tanksley et al. 1992), which was based on F2 L. esculentum × L. pennellii. Thus, any effect of the chromosome 7 inversion would be factored into the reference map, so that the relatively lower recombination observed for the same marker interval in IL 7-4-1 should be due to other factors, such as sequence divergence or genetic background.
The position of introgressed segments within chromosomes appears to play a relatively minor role. Average recombination rates were slightly higher in terminal and pericentric segments than in interstitial and paracentric segments, respectively. However, these trends were not statistically significant. A more pronounced difference was observed between homeologous segments, irrespective of their position within the chromosome, and the substitution lines, which contained intact S. lycopersicoides chromosomes. The latter had much higher recombination rates than observed in introgressed segments of the same wild species chromosomes. These results are consistent with previous research on wheat in which substitution lines containing whole Triticum monococcum chromosomes recombined at higher rates than the segmental lines, but with less than half of the homologous recombination frequency (Luo et al. 2000). Thus, in both wheat and tomato, recombination rates are determined by the level of sequence divergence within a region of homeology and whether it is contained within an intact alien chromosome or a segmental introgression.
Recombination within homeologous vs. homologous regions:
We detected a positive correlation between the rate of homeologous recombination, expressed as a percentage of the expected value, and the length of the introgressed segments on the genetic map of tomato. This correlation could be due to a “preference” for recombination within homologous over homeologous regions of the chromosome. Our data suggest a process whereby chromosomes are scanned for homology and crossovers allocated on the basis of the degree of similarity. Such a process could involve the DNA mismatch repair system, which restricts recombination between homeologous sequences in other model systems, such as Escherichia coli (Zahrt and Maloy 1997), yeast (Datta et al. 1996), and Arabidopsis (Li et al. 2006).
However, our results might also be influenced by purely stochastic processes. Chromosomes with relatively long introgressions contain shorter regions of homology and thus a higher probability of crossovers occurring in the homeologous segments. Another factor that might contribute to the observed correlation is gametic selection. A failure to form at least one crossover, in either a region of homology or a region of homeology, would result in unpaired chromosomes during the first meiotic division. Pairing failure, in turn, would lead to unbalanced gametes, which, in the case of deficiencies, are not viable during gametogenesis of tomato, and, in the case of duplications, would be less competitive during pollination. The result would be to increase the overall frequency of recombinant progeny relative to the rate of crossing over. We previously quantified the rate of pairing failure, as indicated by univalent formation, in heterozygous substitution lines, and found that it had a relatively small effect on recombination estimates (Ji and Chetelat 2003).
Segregation distortion, a common feature of interspecific crosses and their derivatives, can potentially bias recombination estimates in some circumstances (Liu 1998). In practice this has not prevented construction of high-resolution genetic maps in tomato, most of which—due to limited polymorphism in the cultivated genepool—are based on interspecific crosses. We previously described the inheritance of S. lycopersicoides introgressions in progeny of heterozygotes (Canady et al. 2005). For many regions, we observed a deficiency of plants homozygous for the S. lycopersicoides segments, indicating selection against alleles of the wild species during gametogenesis, pollination, and/or zygote development. In this context, recombinant progeny should have a selective advantage since they contain shorter S. lycopersicoides segments, with potentially fewer genes subject to selection. This might contribute to the higher recombination rates observed in long vs. short introgressions. However, the cases of non-Mendelian transmission we observed in the introgression lines could be explained by a small number of segregation distorter loci, no more than one or two per chromosome (Canady et al. 2005). Also, short segments were just as likely as long ones to be subject to these effects. We conclude that the effects of selection might bias some recombination estimates, but probably cannot account for the genomewide recombination reduction or the correlation with segment length that we observed.
Recombination in double- vs. single-introgressed segments:
Our observations of higher recombination rates in lines with intact S. lycopersicoides chromosomes or long introgressed segments led us to construct double-introgression lines containing two segments on opposite chromosome arms. These target/driver genotypes were designed to increase the total length of homeology and thereby reduce the opportunity for homologous recombination. Surprisingly, the compound stocks resulted in only modest increases in recombination within the homeologous segments, much less than values obtained for the corresponding substitution lines. At the same time, recombination in the region of homology between paired S. lycopersicoides segments was greatly enhanced.
One factor that may explain these observations is linkage phase: the target/driver segments were oriented in repulsion phase, in contrast to the substitutions that contain a single S. lycopersicoides chromosome (i.e., all markers in coupling phase). For paired segments in repulsion, the parental chromosomes each contain a single alien introgression and therefore fewer S. lycopersicoides genes that could be under negative selection than gametes that acquire all or part of both homeologous segments as a result of a crossover within either one. On the other hand, recombination in the homologous interval between segments would result in some gametes containing no S. lycopersicoides genetic material, which should have few if any detrimental effects (i.e., reduced linkage drag). This interpretation is consistent with our observations of a bias toward recombinant genotypes that involve a loss of one or both alien segments, relative to those that gained a segment. Target/driver genotypes oriented initially in coupling phase should be less subject to this bias, since a crossover anywhere within or between either segment would produce gametes with less alien genetic material. This prediction could be tested by comparing recombination in coupling- vs. repulsion-phase stocks.
Another factor that may explain the lower recombination in target/driver introgressions than in substitution lines is the presence in the former of an intercalary region of homology between the pair of homeologous segments. This “gap” provides the opportunity for crossovers between perfectly homologous sequences, which would compete against crossovers within the homeologous segments. Assuming that recombination occurs preferentially in regions of homology, then crossover interference would limit the number of recombination events elsewhere on the chromosome, including within either of the introgressed segments. This leads to the prediction that homeologous recombination frequency would be highest if the paired segments cover all or most of the chromosome (i.e., with the shortest possible gap). This is what we observed with the two sets of chromosome 7 target/driver lines.
L. pennellii introgressions can increase recombination in a target region:
Our data on recombination in S. lycopersicoides introgression and substitution lines generally support the hypothesis that homeologous recombination can be enhanced by reducing the opportunity for homologous interaction elsewhere on the same chromosome. Another strategy we explored was to increase the level of sequence homology vis-à-vis the S. lycopersicoides segment by introducing an overlapping introgression from L. pennellii. Recent molecular systematic studies suggest that L. pennellii is a basal taxon in the Lycopersicon clade, phylogenetically intermediate between L. esculentum and S. lycopersicoides (Peralta and Spooner 2001; Spooner et al. 2005). In agreement with the recent molecular phylogenies, the earliest taxonomic descriptions of L. pennellii by Correll (1958) highlighted its distinctive anther morphology, which displays characteristics of both Solanum (lack of a sterile tip) and Lycopersicon (longitudinal dehiscence). It may also be significant that L. pennellii is the only species in the tomato clade that can be experimentally hybridized with both L. esculentum and S. lycopersicoides (Rick 1979). Further evidence of these genetic relationships is that L. pennellii can serve as a “bridge” to overcome the unilateral incompatibility of S. lycopersicoides and facilitate introgression of traits into cultivated tomato (Rick et al. 1988; Chetelat and DeVerna 1991).
For these reasons, we hypothesized that L. pennellii might recombine readily with both the S. lycopersicoides and the L. esculentum chromosomes. In support of this concept, we found that recombination within a S. lycopersicoides segment on chromosome 2 increased nearly 10-fold in the hybrid with L. pennellii. However, in this interspecific cross, the effect of sequence homology within the tested chromosome is confounded by the influence of overall genetic background. These background effects are not insignificant; for example, the chromosomes of L. pennellii recombine readily with those of L. esculentum in the F1 interspecific hybrid, yet once bred into cultivated tomato, recombination between them is greatly reduced (Rick 1969, 1971).
To eliminate the influence of genetic background, we constructed double-introgression lines containing S. lycopersicoides and L. pennellii segments on the same pair of chromosomes (bridging introgressions). We observed that recombination within the S. lycopersicoides segment was substantially elevated within the region of overlap with the L. pennellii segment. Outside the overlap region, recombination was relatively unaffected. These data provide further evidence that the level of sequence homology within the introgressed segment exerts a strong influence on recombination rate, consistent with data from other systems. However, factors unrelated to DNA sequence homology of introgressed segments may also play a role. For example, chromosomes of the parental species differ cytologically in their pericentromeric heterochromatin (Menzel 1962; Khush and Rick 1963), and DNA packaged as heterochromatin is known to be less recombinogenic than euchromatin (Sherman and Stack 1995). Thus differences between the two introgressed segments that affect chromatin packaging or the location of heterochromatin/euchromatin boundaries could influence recombination. Surprisingly, recombination was also enhanced in a double-introgression line that had the L. pennellii and S. lycopersicoides segments on opposite arms of the same chromosome (i.e., no overlap). This result presumably reflects the same processes—either during meiotic recombination or selection during gametophytic or sporophytic phases—that were responsible for similar results from the target/driver genotypes.
Conclusions and outlook:
Several potential practical applications emerge from our studies with the S. lycopersicoides derivatives. First, long introgressed segments or substituted chromosomes are a richer source of recombinants than short ones. Therefore, to reduce linkage drag associated with wild species introgressions, the most expeditious—and somewhat counterintuitive—method would be to start with very large original segments containing the gene of interest, select for single crossovers close to the target locus, and only then generate secondary recombinants on the other side. Sears (1977) made similar recommendations for wheat and pointed out that segments with crossovers on opposite sides could be allowed to recombine, resulting in the shortest possible introgressions. Second, recombination within a homeologous segment can be increased by constructing a double introgression with greater homology. In the present experiments, segments from L. pennellii recombined readily with both S. lycopersicoides and L. esculentum; however, we have not tested similar stocks from other wild relatives. Thus, the source of the bridging segments might be manipulated to maximize recombination frequency. Third, the S. lycopersicoides segments could be used to suppress recombination on one arm of a chromosome to increase crossover frequency within a target introgression (e.g., from more closely related species) on the other arm.
A significant disadvantage of these chromosome engineering strategies is the extra time involved in constructing compound stocks and then eliminating residual genetic material in later generations. Situations that might justify the extra effort include genes located in regions of suppressed recombination (e.g., near centromeres) or map-based cloning projects where the ordering of genes, not variety development, is the primary goal. In other cases, a “brute force” screening for rare recombinants using high-throughput marker technologies might be more efficient.
Acknowledgments
The authors are grateful to the students and staff of the Tomato Genetics Resource Center (TGRC) for providing seeds and maintaining plants. Dani Zamir donated the L. pennellii introgression lines to the TGRC, and Steve Tanksley provided probes. Sheh May Tam assisted with sequence analysis. Kevin Alpert, Charley Rick, and Jan Dvorak shared creative insights during the research, and two reviewers provided constructive comments on the manuscript. This research was supported in part by grants from the California Tomato Commission and the U.S. Department of Agriculture, National Research Initiative (nos. 91-37300-6382 and 99-35300-7683).
References
- Akhunov, E. D., A. R. Akhunova, A. M. Linkiewicz, J. Dubcovsky, D. Hummel et al., 2003. Synteny perturbations between wheat homoeologous chromosomes caused by locus duplications and deletions correlate with recombination rates. Proc. Natl. Acad. Sci. USA 100: 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert, K. B., and S. D. Tanksley, 1996. High-resolution mapping and isolation of a yeast artificial chromosome contig containing fw2.2: a major fruit weight quantitative trait locus in tomato. Proc. Natl. Acad. Sci. USA 93: 15503–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canady, M. A., V. Meglic and R. T. Chetelat, 2005. A library of Solanum lycopersicoides introgression lines in cultivated tomato. Genome 48: 685–697. [DOI] [PubMed] [Google Scholar]
- Chetelat, R. T., and J. W. Deverna, 1991. Expression of unilateral incompatibility in pollen of Lycopersicon pennellii is determined by major loci on chromosomes 1, 6 and 10. Theor. Appl. Genet. 82: 704–712. [DOI] [PubMed] [Google Scholar]
- Chetelat, R. T., V. Meglic and P. Cisneros, 2000. A genetic map of tomato based on BC1 Lycopersicon esculentum × Solanum lycopersicoides reveals overall synteny but suppressed recombination between these homeologous genomes. Genetics 154: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll, D. S., 1958. A new species and some nomenclatural changes in Solanum, section Tuberarium. Madrono 14: 232–238. [Google Scholar]
- Datta, A., A. Adjiri, L. New G. F. Crouse and S. Jinks-Robertson, 1996. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccharomyces cerevisae. Mol. Cell. Biol. 16: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverna, J. W., C. M. Rick, R. T. Chetelat, B. J. Lanini and K. B. Alpert, 1990. Sexual hybridization of Lycopersicon esculentum and Solanum rickii by means of a sesquidiploid bridging hybrid. Proc. Natl. Acad. Sci. USA 87: 9486–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vicente, M. C., and S. D. Tanksley, 1991. Genome-wide reduction in recombination of backcross progeny derived from male versus female gametes in an interspecific backcross of tomato. Theor. Appl. Genet. 83: 173–178. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., and D. Zamir, 1995. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141: 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman, E., F. Carrari, Y. S. Liu, A. R. Fernie and D. Zamir, 2004. Zooming in on a quantitative trait for tomato using interspecific introgressions. Science 305: 1786–1789. [DOI] [PubMed] [Google Scholar]
- Ji, Y., and R. T. Chetelat, 2003. Homeologous pairing and recombination in Solanum lycopersicoides monosomic addition and substitution lines of tomato. Theor. Appl. Genet. 106: 979–989. [DOI] [PubMed] [Google Scholar]
- Ji, Y., R. A. Pertuze and R. T. Chetelat, 2004. Genome differentiation by GISH in interspecific and intergeneric hybrids of tomato and related nightshades. Chromosome Res. 12: 107–116. [DOI] [PubMed] [Google Scholar]
- Kaloshian, I., J. Yaghoobi, T. Liharska, J. Hontelez, D. Hanson et al., 1998. Genetic and physical localization of the root-knot nematode resistance locus Mi in tomato. Mol. Gen. Genet. 257: 376–385. [DOI] [PubMed] [Google Scholar]
- Khush, G. S., and C. M. Rick, 1963. Meiosis in hybrids between Lycopersicon esculentum and Solanum pennellii. Genetica 33: 167–183. [Google Scholar]
- Lander, E. S., P. Green, J. Abrahamson, A. Barlow, M. J. Daly et al., 1987. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181. [DOI] [PubMed] [Google Scholar]
- Li, L., M. Jean and F. Belzile, 2006. The impact of sequence divergence and DNA mismatch repair on homeologous recombination in Arabidopsis. Plant J. 45: 908–916. [DOI] [PubMed] [Google Scholar]
- Liu, B. H., 1998. Statistical Genomics: Linkage, Mapping, and QTL Analysis. CRC Press, Boca Raton, FL, pp. 199–204.
- Liu, Y.-S., and D. Zamir, 1999. Second generation L. pennellii introgression lines and the concept of bin mapping. Tomato Genet. Coop. Rep. 49: 26–30. [Google Scholar]
- Luo, M. C., Z. L. Yang, R. S. Kota and J. Dvorak, 2000. Recombination of chromosomes 3Am and 5Am of Triticum monococcum with homeologous chromosomes 3A and 5A of wheat: the distribution of recombination across chromosomes. Genetics 154: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel, M. Y., 1962. Pachytene chromosomes of the intergeneric hybrid Lycopersicon esculentum x Solanum lycopersicoides. Am. J. Bot. 49: 605–615. [Google Scholar]
- Menzel, M. Y., 1964. Differential chromosome pairing in allotetraploid Lycopersicon esculentum-Solanum lycopersicoides. Genetics 50: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte, A. J., and S. D. Tanksley, 2000. Fine mapping of a quantitative trait locus (QTL) from Lycopersicon hirsutum chromosome 1 affecting fruit characteristics and agronomic traits: breaking linkage among QTLs affecting different traits and dissection of heterosis for yield. Theor. Appl. Genet. 100: 471–479. [Google Scholar]
- Nesbitt, T. C., and S. D. Tanksley, 2002. Comparative sequencing in the genus Lycopersicon: implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta, I. E., and D. M. Spooner, 2001. Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. Section Lycopersicon [Mill.] Wettst. Subsection Lycopersicon). Am. J. Bot. 88: 1888–1902. [PubMed] [Google Scholar]
- Pertuze, R. A., Y. Ji and R. T. Chetelat, 2002. Comparative linkage map of the Solanum lycopersicoides and S. sitiens genomes and their differentiation from tomato. Genome 45: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Pillen, K., O. Pineda, C. B. Lewis and S. D. Tanksley, 1996. Status of genome mapping tools in the taxon Solanaceae, pp. 282–308 in Genome Mapping in Plants, edited by A. H. Paterson. R. G. Landes, Austin, TX.
- Rick, C. M., 1951. Hybrids between Lycopersicon esculentum Mill. and Solanum lycopersicoides Dun. Proc. Natl. Acad. Sci. USA 37: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick, C. M., 1969. Controlled introgression of chromosomes of Solanum pennellii into Lycopersicon esculentum: segregation and recombination. Genetics 62: 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick, C. M., 1971. Further studies on segregation and recombination in backcross derivatives of a tomato species hybrid. Biol. Zentralbl. 90: 209–220. [Google Scholar]
- Rick, C. M., 1979. Biosystematic studies in Lycopersicon and closely related species of Solanum, pp. 667–678 in The Biology and Taxonomy of the Solanaceae, edited by J. G. Hawkes, R. N. Lester and A. D. Skelding. Academic Press, New York.
- Rick, C. M., J. W. Deverna, R. T. Chetelat and M. A. Stevens, 1986. Meiosis in sesquidiploid hybrids of Lycopersicon esculentum and Solanum lycopersicoides. Proc. Natl. Acad. Sci. USA 83: 3580–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick, C. M., R. T. Chetelat and J. W. Deverna, 1988. Recombination in sesquidiploid hybrids of Lycopersicon esculentum x Solanum lycopersicoides and derivatives. Theor. Appl. Genet. 76: 647–655. [DOI] [PubMed] [Google Scholar]
- Sears, E. R., 1977. Analysis of wheat-Agropyron recombinant chromosomes, pp. 63–72 in Interspecific Hybridization in Plant Breeding, edited by E. Sanchez-Monge and F. Garcia-Olmedo. Proceedings of the Eighth Congress of EUCARPIA, Escuela Técnica Superior de Ingenieros Agrónomos, Madrid.
- Shen, P., and H. V. Huang, 1986. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112: 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, J. D., and S. M. Stack, 1995. Two-dimensional spreads of synaptonemal complexes from solanaceous plants. VI. High resolution recombination nodule map for tomato (Lycopersicon esculentum). Genetics 141: 683–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, D. M., I. E. Peralta and S. Knapp, 2005. Comparison of AFLPs with other markers for phylogenetic inference in wild tomatoes. [Solanum L. section Lycopersicon (Mill.) Wettst.]. Taxon 54: 43–61. [Google Scholar]
- Stebbins, G. L., 1958. The inviability, weakness, and sterility of interspecific hybrids. Adv. Genet. 9: 147–215. [DOI] [PubMed] [Google Scholar]
- Suiter, K. A., J. F. Wendel and J. S. Case, 1983. LINKAGE-1: a PASCAL computer program for the detection and analysis of genetic linkage. J. Hered. 74: 203–204. [DOI] [PubMed] [Google Scholar]
- Tanksley, S. D., M. W. Ganal, J. P. Prince, M. C. De Vicente, M. W. Bonierbale et al., 1992. High density molecular linkage maps of the tomato and potato genomes. Genetics 132: 1141–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Knaap, E., A. Sanyal, S. A. Jackson and S. D. Tanksley, 2004. High-resolution fine mapping and fluorescence in situ hybridization analysis of sun, a locus controlling tomato fruit shape, reveals a region of the tomato genome prone to DNA rearrangements. Genetics 168: 2127–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen, J. M., J. M. Sandbrink, M. Vrielink, R. Verkerk, P. Zabel et al., 1994. An RFLP linkage map of Lycopersicon peruvianum. Theor. Appl. Genet. 89: 1007–1013. [DOI] [PubMed] [Google Scholar]
- Van Wordragen, M. F., R. L. Weide, E. Coppoolse, M. Koornneef and P. Zabel, 1996. Tomato chromosome 6: a high resolution map of the long arm and construction of a composite integrated marker-order map. Theor. Appl. Genet. 92: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Zahrt, T. C., and S. Maloy, 1997. Barriers to recombination between closely related bacteria: MutS and RecBCD inhibit recombination between Salmonella typhimurium and Salmonella typhi. Proc. Natl. Acad. Sci. USA 94: 9786–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, D., 2001. Improving plant breeding with exotic genetic libraries. Nat. Rev. 2: 983–989. [DOI] [PubMed] [Google Scholar]