Abstract
Lipoxins (LXs), endogenously produced eicosanoids, possess potent anti-inflammatory, proresolution bioactivities. We investigated the potential of LXA4 (1 to 10 nmol/L) to modify the effects of platelet-derived growth factor (PDGF)-induced gene expression in human renal mesangial cells (hMCs). Using oligonucleotide microarray analysis we profiled profibrotic cytokines and matrix-associated genes induced in response to PDGF. LXA4 modulated the expression of many PDGF-induced genes, including transforming growth factor-β1, fibronectin, thrombospondin, matrix metalloproteinase 1, and several collagens. Analysis of both transcript and protein levels confirmed these findings. Because the activated glomerulus is frequently a source of injurious mediators that contribute to tubulointerstitial damage, we investigated the effect of hMC-secreted products on the integrity of renal proximal tubular epithelial cells using an in vitro model of progressive renal disease. Cell supernatant from PDGF-stimulated hMCs caused morphological and genetic changes in proximal tubular epithelial cells, consistent with a profibrotic phenotype. Interestingly, supernatant from cells pre-exposed to LXA4 and PDGF did not induce these effects. These results suggest a novel role for LXA4 as a potent modulator of matrix accumulation and profibrotic change and suggest a potential protective role in progressive renal disease.
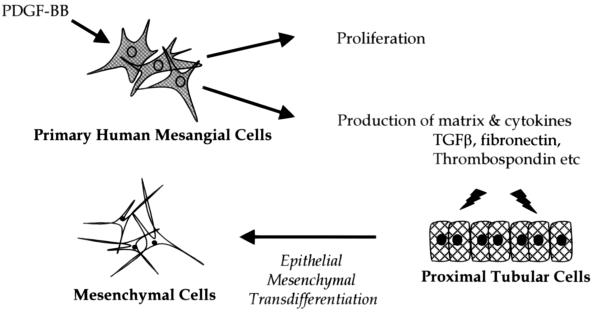
Cellular proliferation, migration, and extracellular matrix expansion are fundamental responses of mesangial cells to glomerular injury. The affected glomerulus initiates a host of reactions involving the release of proinflammatory mediators including proteases, extracellular matrix products, eicosanoids, and growth factors. Resolution of such appropriate host defense is a dynamically regulated process that may be subverted in chronic inflammation.1 Sustained inflammatory and growth factor responses result in the development of progressive glomerular diseases characterized by tubulointerstitial fibrosis. Although the mechanisms underlying these changes are incompletely understood, many studies have implicated a major role for cytokines and growth factors including platelet-derived growth factor (PDGF), basic fibroblast growth factor, and transforming growth factor (TGF)-β1.2,3
PDGF is implicated in the progression of renal disease, being synthesized by infiltrating macrophages and platelets, as well as resident mesangial cells in response to multiple stimuli including endothelin, thrombin, and angiotensin II.3 PDGF bioactivity encompasses increased cellular proliferation,4 mesangial matrix expansion,5 and increased expression of the profibrotic cytokine TGF-β1.6,7 The roles of both PDGF and TGF-β1 in renal disease have been well characterized. However, current therapeutic interventions to regulate chronic renal inflammation are limited and PDGF has been proposed as a potential therapeutic target.8,9
There is growing evidence that lipoxins (LXs), endogenously produced eicosanoids, may have significant anti-inflammatory and proresolution bioactions. Biphasic lipid mediator production has been demonstrated in the context of an effective host defense. Initial proinflammatory mediator production is superseded by the production of anti-inflammatory, proresolution mediators including LXs, resolvins, and docosatrienes.10,11 LXs are well documented to inhibit neutrophil chemotaxis, adhesion, and transmigration12 and to stimulate monocyte chemotaxis.13 More recent evidence of their anti-inflammatory roles includes modulation of eosinophil activation.14 A role for LX promoting the resolution of inflammation has been demonstrated by stimulating phagocytic clearance of apoptotic neutrophils in vivo15 and in vitro.12 In the context of renal inflammation we have previously reported that human mesangial cells (hMC) express a G-protein-coupled receptor that binds LXA4, known as the ALXR.16 We have reported LXA4 inhibition of PDGF and epidermal growth factor (EGF)-induced hMC mitogenesis.4,6 These effects are mediated by modulation of receptor activation and inhibition of specific downstream signaling pathways, including the Akt/PKB pathway.6 Given the ability of LXA4 to inhibit the effects of PDGF receptor activation in hMCs, it was of further interest to elucidate the effects of LXA4 on the induction of PDGF-mediated gene expression changes. Here we report that PDGF stimulates the expression of multiple genes associated with matrix expansion and fibrosis, and that LXA4 modulates PDGF-induced gene expression. We report that soluble factors released by PDGF-stimulated hMCs can induce a profibrotic response in renal epithelia characterized by epithelial-to-mesenchymal transformation (EMT). The equivalent profibrotic response was prevented in supernatants from hMCs treated with LXA4 and PDGF. These data suggest that LXA4 may have distinct anti-fibrotic actions in human renal disease.
Materials and Methods
Materials
LXA4 was obtained from Biomol (Plymouth Meeting, PA). Human recombinant PDGF-BB was acquired from Upstate Biotechnology, Milton Keynes, UK. Anti-thrombospondin and anti-fibronectin monoclonal antibodies were from Calbiochem (Nottingham, UK). Enzyme-linked immunosorbent assay (ELISA) kits for TGF-β1 and matrix metalloproteinase (MMP)-1 were from R&D (Abingdon, Oxon, UK) and Amersham (Buckinghamshire, UK) respectively. Anti-α-smooth muscle actin monoclonal antibody was from Sigma-Aldrich (Tallaght, Dublin, Ireland) and anti-E-cadherin monoclonal antibody was obtained from BD Biosciences (Oxford, UK), alternatively, for immunoblotting anti-E-cadherin (U3254) from Sigma-Aldrich was used. Fluorescein isothiocyanate-conju-gated phalloidin was obtained from Molecular Probes (Eugene, OR).
Mesangial Cell Culture
hMCs were isolated from a nephrectomy sample obtained from the Mater Misercordiae University Hospital in accordance with institutional ethical guidelines. As previously described by Mitchell and colleagues,6 a sample of cortex was isolated and differentially sieved to extract the glomeruli, which were subsequently grown on collagen-coated plates. Cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), which was selective for mesangial cell growth. These cells retained the phenotypic characteristics of hMCs, including stellate morphology, positive staining for vimentin and α-smooth muscle actin, and negative staining for ZO-1 and occludin.17
In Vitro Model of EMT
Murine cortical tubular (MCT) cells were grown in Dulbecco’s modified Eagle’s medium-F12 Hams supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) and were stimulated in Dulbecco’s modified Eagle’s medium-F12 Hams supplemented with insulin-transferrin-selenium supplement (Sigma), l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and hydrocortisone (K1 media). Supernatant from hMCs treated with vehicle, LXA4 (1 nmol/L), PDGF (10 ng/ml), or LXA4 (15 minutes) pretreatment followed by PDGF were removed after 24 hours. MCT cells were stimulated with supernatants from pre-exposed hMCs, diluted in K1 media (Figure 1). To control for variability in mesangial cell number in PDGF versus vehicle-treated cells, supernatants were diluted accordingly. PDGF treatment was associated with a twofold increase in cell number as compared to vehicle, therefore conditioned media was diluted 1:3 with K1 media. Vehicle-treated supernatant was alternatively diluted 1:1 with K1 media.
Figure 1.
Experimental model of hMC-induced changes in MCT cells.
Oligonucleotide Microarray Analysis
hMCs were serum restricted (RPMI 1640 supplemented with 0.2% fetal calf serum) for 48 hours before serum starving (RPMI 1640 supplemented with 0% fetal calf serum) for a further 1 hour before stimulating. Cells were subsequently treated with PDGF (10 ng/ml) ± LXA4 (1 nmol/L) in 0% fetal calf serum-RPMI 1640 for 24 hours. RNA from three independent experiments was pooled after isolation using lysis buffer, in accordance with the Qiagen minicolumn preparation (Qiagen, Valencia, CA). Complementary DNA (cDNA) synthesis, in vitro transcription, and microarray analysis were performed as we have previously reported.18 Briefly, cDNA was synthesized from total RNA using the Superscript Choice kit (Invitrogen, Carlsbad, CA). Biotin-labeled cRNA prepared from template cDNA was fragmented and hybridized to Affymetrix HGU133A arrays according to the Affymetrix protocol (Affymetrix, Santa Clara, CA). Arrays were then fluorescently labeled before scanning with a confocal scanner (Affymetrix). This process was repeated to obtain a duplicate chip from which data were compiled and analyzed.
Image files were obtained through Affymetrix GeneChip software (MAS5). Robust multichip analysis (RMA) was subsequently performed. RMA is a technique that analyzes directly from the Affymetrix microarray and is comprised of three steps; background adjustment, quantile normalization, and summarization. RMAexpress was used to make the data accessible to a Microsoft Windows operating system for further analysis, as per Sadlier and colleagues.19
The data from each microarray were collected and expression data for each condition were compared to control. Genes altered by PDGF, causing a 0.5 signal log ratio (SLR) or greater change, with respect to control on both microarrays (equivalent to a fold change in expression of 1.4 or greater) were termed significant and further analyzed for differential expression. Using unsupervised hierarchical cluster analysis as described in Eisen and colleagues20 a visual representation of genomic differential expression was attained. Furthermore, genes could be categorized using a web-based ontology program (Onto-Express).21 This program assigns genes a category based on current known biological function, however redundancy of genes between several categories may exist.
Promoter and Transcription Factor Analysis
Genes significantly altered by PDGF were analyzed using the web-based software Genomatix (Genomatix Software GmbH, Munich, Germany). Genes mapped to loci with experimentally verified promoter regions were further analyzed for common transcription factor binding sites.22 A random expectation value (re-value) was assigned to each transcription factor (the program assigns an expectation value for the number of transcription factor binding site matches per 1000 bp of random DNA sequence). The actual occurrence and random expectation of a given transcription factor were compared to confirm the presence of a binding site. Furthermore, we examined the binding of stimulating protein 1 (SP-1) to consensus SP-1 binding sites using the TransAM SP-1 kit (Active Motif, Rixensart, Belgium). hMCs were treated with PDGF (10 ng/ml) ± LXA4 (1 nmol/L) for 24 hours before extraction of nuclear lysate. Ten μg of nuclear lysate protein was added to each well and the assay was conducted according to the manufacturer’s protocol.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Quantitative real-time PCR was performed using TaqMan universal PCR master mix (P/N 4304437; Applied Biosystems, Weiterstadt, Germany) as per the manufacturer’s protocol. Samples were run in duplicate and were analyzed using the ABI Prism 7700 sequence detection system (Applied Biosystems). TaqMan PCR reactions were multiplexed for the gene of interest and VIC-labeled 18S rRNA, as an endogenous control (P/N 4310893E, Applied Biosystems). FAM-labeled forward primers, reverse primers, and TaqMan probes are listed below (Table 1).
Table 1.
Primer Sequences for Quantitative RT-PCR
| Primer | Forward | Reverse | Probe |
|---|---|---|---|
| Human fibronectin | CCGCCGAATGTAGGACAAGA | TGCCAACAGGATGACATGAAA | CAACCATCTCATGGGCCCCATTCC |
| Human collagen I α I | TGTTGGCCCAAGAGGTCCT | CACCGGGCTCTCCCTTATC | TGGCCCACAAGGCATTCGTGG |
| Rat fibronectin | AAACAGGTCTGGACTCCCCA | CAGAATGCTCGGCGTGATG | TCTTCTGATGTCACCGCCAACTCATTCA |
| Mouse VEGF | AACGATGAAGCCCTGGAGTG | TTGATCCGCATGATCTGCAT | TGCCCACGTCAGAGAGCAACATCAC |
| Mouse CTGF | CCCACACAAGGGCCTCTTC | CCATCTTTGGCAGTGCACAC | CCCCCGCCAACCGCAAGATT |
Western Blot Analysis
hMCs were treated as follows; cells were serum-starved for 24 hours before the addition of stimuli, they were then pretreated with LXA4 (1 nmol/L) or vehicle for 15 minutes before addition of PDGF (10 ng/ml). The treatment was for the indicated time periods (24 to 72 hours). Supernatants were retained and lysates were harvested in RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris, 5 mmol/L ethylenediamine tetraacetic acid, 10 mmol/L NaF, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate). The lysates were clarified by centrifugation at 10,621 × g for 10 minutes, the supernatant fraction was retained, and the protein concentration quantified using a Bradford protein assay. MCT cells were conditioned in K1 media, TGF-β1 (10 ng/ml) and EGF (10 ng/ml) were added as a positive control for EMT. MCT cells were treated with pretreated hMC supernatant, the volume of supernatant from each condition was determined by hMC number. Cells were subsequently harvested as above and protein levels were determined by the Bradford assay. Specific protein levels were detected by immunoblotting lysates from hMC or MCT cell culture.
For Western blot analysis, 20 to 40 μg of hMC protein or 30 μg of MCT protein were loaded into each lane under reducing conditions on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and subsequently transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) by electroblotting. Nonspecific antibody binding was reduced by blocking the membrane in 3% bovine serum albumin (Sigma, Dublin, Ireland) for 1 hour at room temperature. The antibody was then added at a concentration according to the manufacturer’s instructions and incubated on a rocking platform at 4°C, overnight. Membranes were subsequently incubated with a horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature and visualized using enhanced chemiluminescence (Santa Cruz, Heidelberg, Germany) and X-ray film. To check for equal loading the membranes were stripped using sodium dodecyl sulfate, blocked and reprobed with anti-β-actin monoclonal antibody (Sigma).
Quantitaion of TGF-β1 Production
Quiescent hMCs were treated as indicated and at 72 hours the supernatant was retained and assayed for TGF-β1 release by ELISA (R&D Systems) as per manufacturer’s protocol.
Quantitaion of MMP-1 Production
Quiescent hMCs were treated as above, at 6, 18, 24, 48 and 72 hours time-points the supernatant was retained and assayed for MMP-1 release by ELISA (Amersham Biosciences, Bucks, UK) as per the manufacturer’s protocol.
Immunofluorescent Microscopy
MCT cells were cultured on chamber slides (Nalge Nunc, Naperville, IL), and subsequently stimulated with pretreated hMC supernatant, as detailed above. Cells were then washed and fixed with 4% paraformaldehyde added directly to the wells. After washing in phosphate-buffered saline (PBS), cells were permeabilized in 0.5% Triton-X for 10 minutes, blocking agent (PBS containing 1% goat serum and 3% bovine serum albumin) was then added. Cells were kept on a rocking platform for 1 hour at room temperature. The primary antibody was subsequently added at concentrations according to the manufacturer’s instructions and incubated overnight on a rocking platform at 4°C. After washing, a fluorescein isothiocyanate-conjugated secondary antibody, Goat anti-mouse (Molecular Probes) was added at room temperature for 1 hour, after this, cells were washed in PBS containing 4,6-diamidino-2-phenylindole (DAPI) stain for nuclei visualization. Slides were then mounted with coverslips and analyzed using phase contrast (×40 magnification) and also using Axiovert 200 fluorescent microscopy DAPI and fluorescein isothiocyanate filters (Carl Zeiss, Jena, Germany).
Results
PDGF Induces Expression of a Distinct Cohort of Genes in hMCs
Differential gene expression was examined using oligonucleotide microarrays (HGU133A), cDNA was extracted from hMCs exposed to 10 ng/ml PDGF for 24 hours with or without LXA4 pretreatment (15 minutes, 1 nmol/L). This procedure allowed whole genome analysis of hMCs in normal and growth factor-treated cells, identifying signatures of the conditions.20 Data obtained from each condition were normalized with respect to control (ie, vehicle-stimulated cells). An overview of the information acquired is represented in Figure 2A, PDGF significantly altered (0.5 SLRs or greater) 4.16% of genes represented on the array (926 of 22,283 genes). Consistent with the modulation of PDGF signaling, pretreatment with LXA4 diminished the effects of PDGF. LXA4 pretreatment of the PDGF-stimulated response prevented significant PDGF responses in almost 40% of the 926 genes. Of the 582 genes increased significantly by PDGF, pretreatment of hMCs with LXA4 diminished this response to 353 genes stimulated by PDGF. Genes decreased by PDGF consisted of 344 transcripts, again LXA4 pretreatment diminished this to 205 genes. To gain more insight into the significance of these changes, genes are given functionality based on current known biological process using Onto-Express,21 a web-based ontological program (Figure 2B). Categories include embryogenesis, cell proliferation, fibrosis-related, immune response, and lipid metabolism. LXA4 decreased the number of PDGF-driven genes, with particular attention to cell proliferation (42% reduction) and fibrosis-related (49% reduction).
Figure 2.
Global changes in gene expression in hMCs, representative ontological data examining biological functionality, and transcription factor analysis of genes. Gene expression changes in hMCs treated with PDGF (10 ng/ml) alone or PDGF with LXA4 (1 nmol/L) pretreatment were analyzed. Three independent experiments were pooled for hybridization to each microarray. Duplicate experiments using HGU133A Affymetrix microarrays are illustrated, data are representative of genes differentially expressed by 0.5 SLRs or greater (≥0.5 SLR and ≤0.5 SLR) from the average of duplicate arrays. A: An overview of the numbers of differentially expressed genes. B: Changes in gene expression within major functional families using OntoExpress. C: Unsupervised hierarchical cluster analysis of the average of duplicate arrays and ontological clustering. D: Transcription factor consensus binding sequences on PDGF-altered genes, random-expectation values, and actual binding site occurrence are shown. E: SP-1 expression in hMC nuclear lysate after treatment with PDGF (10 ng/ml), LXA4 (1 nmol/L), vehicle, or LXA4 (15 minutes) followed by PDGF. Data are mean ± SEM of duplicate measurements from three independent experiments (*P < 0.05 compared to vehicle, #P < 0.05 compared to PDGF).
Data obtained from RMA analysis of the oligonucleotide microarray were organized using pairwise average linkage clustering that arranges data into unsupervised hierarchical clusters, whereby each gene represented is equally weighted. This was achieved using Eisen-Lab20 web-based cluster software (Figure 2C). Genes significantly altered by PDGF (less than or equal to 0.5 SLR and greater than and equal to 0.5 SLR) were represented. Genome-wide data were clustered using statistical algorithms to arrange genes according to similarity in patterns of expression with co-expressed genes believed to have functional similarity.20 The primary cluster represented all genes significantly altered by PDGF, normalized with respect to control. Additional to this, each ontological category was clustered. The cell proliferation cluster displayed 65 transcripts, of these, 36 were significantly altered in the presence of LXA4, including various cyclins and p53-induced proteins. Of the 35 fibrosis transcripts, 18 were significantly altered by LXA4 pretreatment. Fibronectin, thrombospondin, and TGF-β1 were among these. Immune response, embryogenesis, and lipid metabolism clusters also displayed altered transcript expression with LXA4 pretreatment.
Of particular interest to us was whether we could define specific transcription factors (TFs) activated in hMCs when exposed to PDGF and whether these might further be subdivided into LXA4-regulated and LXA4-independent genes. Analysis of gene promoter regions was conducted using Genomatix22 software. Genes significantly affected by PDGF were mapped to loci containing experimentally verified promoter regions. Subsequently, common TF binding regions were extracted using MatInspector software. Several ubiquitously expressed transcription factor-binding sites were found including cAMP response element binding site (CREB), early growth response factor (EGRF), and stimulating protein 1 (SP-1) (Figure 2D). SP-1 was found to be present on almost half of all PDGF-stimulated genes. Each TF is assigned a random-expectation (re) value based on the estimated number of binding sites for that TF per 1000 bp of sequence. For the SP-1 TF, the re-value was predicted at 1.7, however actual occurrence was averaged at 2.8. Subsequently, we analyzed SP-1 TF binding using a TransAM assay that measures SP-1 and posttranslationally modified SP-1. We found that PDGF significantly elevated levels of SP-1 (1.6-fold) and that this response was attenuated with LXA4 pretreatment (1.18-fold) (Figure 2E).
Validation of Oligonucleotide Microarray Findings
A cohort of genes was selected for further analysis to validate gene expression changes identified by oligonucleotide microarray. In Figure 3a quantitative RT-PCR data displayed PDGF-induced (10 ng/ml) increases in the expression of fibronectin and collagen type I α1 transcripts at 24 hours normalized with respect to 18S-ribosomal RNA (1.2- and 1.7-fold induction, respectively). Pretreatment with LXA4 (1 nmol/L) prevented PDGF-induced gene expression increases. PDGF also induced alterations in the protein levels of fibronectin, decorin, and thrombospondin (Figure 3b). Stimulation of mesangial cells with PDGF for 24 hours resulted in elevated levels of fibronectin and thrombospondin, a TGF-β1 activator.23 Corresponding decreases were observed of the small proteoglycan decorin, a known inhibitor of the profibrotic cytokine TGF-β1.24 These changes in protein expression were consistent with gene expression array findings and were altered with the pretreatment of hMCs with LXA4 (1 nmol/L).
Figure 3.
Altered expression levels of hMC DNA, protein, and secreted protein in response to PDGF ± LXA4, validating changes demonstrated by Affymetrix microarray. hMCs were treated with PDGF (10 ng/ml), LXA4 (1 nmol/L), vehicle, or LXA4 (15 minutes) followed by PDGF. a: Changes in the expression of collagen I αI and fibronectin cDNA after 24 hours, as measured by real-time PCR. The average of two independent experiments performed in duplicate is shown. b: Altered levels of fibronectin, thrombospondin 1, and decorin protein measured by immunoblotting after 24 hours, corresponding β-actin blots demonstrate equal loading. Data are representative of three independent experiments. c: TGF-β1 and MMP-1 secretion from hMCs as measured by ELISA at the indicated time points. Data shown are mean ± SEM of duplicate measurements from three independent experiments (*P < 0.05, **P < 0.005).
Transforming growth factor (TGF)-β1 and MMP-1 secretion from hMCs was also measured in response to PDGF (10 ng/ml) with or without LXA4 (1 nmol/L) (Figure 3c). Supernatant from stimulated cells was collected at the indicated time points and assayed using ELISA. TGF-β1 levels were increased significantly by PDGF at 72 hours (2.1-fold change) with LXA4 pretreatment ablating this effect (1.1-fold change). Consistent with this, secreted MMP-1 levels were significantly enhanced in response to PDGF at 6, 18, and 24 hours, (1.2-, 1.5-, and 1.6-fold change, respectively) whereas LXA4 achieved significant diminution of the PDGF effect at 6 and 18 hours only (0.9- and 0.7-fold change).
Epithelial-to-Mesenchymal Transformation of Proximal Tubular Epithelial Cells
It has been proposed that pleiotropic mediators released from the inflamed glomerulus play an important role in progressive renal disease by tubulointerstitial fibrosis.25,26 Tubulointerstitial fibrosis is frequently characterized by EMT. Consistent with this we observed that supernatants from hMCs treated with PDGF (10 ng/ml, 24 hours) induced a loss of E-cadherin expression concomitant with increased levels of α-smooth muscle actin in MCT cells (Figure 4b). These phenotypic changes mirrored those seen with the prototypic inducers of EMT, TGF-β1, and EGF (Figure 4a).27 MCT cells treated with cell supernatant from vehicle or LXA4-treated hMCs retained much of their epithelial phenotype. Remarkably, this observed change in MCT phenotype was less substantial after treatment with supernatant from PDGF-stimulated and LXA4-pretreated hMCs. Pretreatment with LXA4 diminished the effects of PDGF thereby impeding the indicated transformation. These changes were verified using Western blot analysis of MCT lysate (Figure 4c). PDGF-stimulated hMC supernatant caused decreases in E-cadherin and corresponding increases in α-SMA levels. Again, supernatant from hMCs pretreated with LXA4 diminished this effect.
Figure 4.
Altered morphology and expression in MCT cells in response to pretreated hMC supernatant. a: Immunocytochemistry of MCT cells was used to illustrate altered expression in E-cadherin and α-SMA after the induction of EMT by TGF-β1 + EGF treatment throughout 72 hours. b: Changes in MCT cells after 72 hours of incubation with pretreated hMC supernatant, measured by phalloidin, E-cadherin, and α-SMA. hMCs were stimulated throughout a 24-hour period with PDGF (10 ng/ml), LXA4 (1 nmol/L), vehicle or LXA4 (15 minutes) followed by PDGF. Cell nuclei are DAPI stained (blue) and antibodies are fluorescein isothiocyanate conjugated (green). c: MCT cells were treated as indicated above, whole cell lysates were extracted from three independent experiments. Immunoblots for E-cadherin, α-SMA, and β-actin are displayed.
Changes in MCT gene expression of fibronectin, vascular endothelial growth factor (VEGF), and connective tissue growth factor (CTGF) were measured using quantitative real-time RT-PCR. The positive control (TGF-β1 and EGF) displayed significant increased expression of each gene, as compared to K1 media alone (Figure 5A). Consistent with this, supernatant from PDGF-stimulated (10 ng/ml, 24 hours) hMCs caused an increase in the expression of VEGF, CTGF, and fibronectin in MCT cells. Supernatant from hMCs pretreated with LXA4 (1 nmol/L, 15 minutes) and subsequently PDGF-stimulated, diminished these observed changes in gene expression (Figure 5; B, C, and D), consistent with LXA4 modulation of PDGFR signals. Expression of all three genes was continuously decreased with LXA4-pretreated supernatant. Changes in epithelial cell gene expression induced by hMC supernatants were not due to residual PDGF in the media. This was verified by the addition of the tyrophostin PDGFR kinase inhibitor AG1296 (10 nmol/L) to hMC supernatant. Addition of the inhibitor did not significantly effect observed gene expression changes (data not shown). Furthermore, direct stimulation of the MCT cells with PDGF-BB did not induce significant expression of fibronectin, VEGF, or CTGF (Figure 5A).
Figure 5.
Altered fibronectin, VEGF, and CTGF gene expression levels in MCT cells, as measured by quantitative real-time PCR. A: Differences in fibronectin, VEGF, and CTGF mRNA in MCT cells treated with a combination of TGF-β1 and EGF or PDGF-BB for 72 hours. B–D: Altered mRNA levels of fibronectin, VEGF, and CTGF, respectively. RNA was extracted from MCT cells treated with pretreated hMC supernatant for 72 hours as previously indicated. Results are displayed as normalized relative quantity, data are mean ± SEM of duplicate measurements from three independent experiments (*P < 0.05 compared to vehicle, #P < 0.05 compared to PDGF). All values have been normalized to respective 18s rRNA.
Discussion
The pathology of progressive renal disease in glomerulonephritis (GN) involves mesangial cell proliferation and migration, matrix expansion, and cytokine production, which left unchecked may result in end-stage renal disease characterized by tubulointerstitial fibrosis. Initial insult to the glomerulus causes a host of inflammatory reactions resulting in the progressive accumulation of extracellular matrix components, thus decreasing filtration surface area.28 The release of inflammatory mediators and cytokines/growth factors into the surrounding interstitium accompanies the development of fibrosis and tubular atrophy. Previous reports have suggested the involvement of PDGF in mediating these effects.7,29
LXs have been shown to exert potent anti-inflammatory actions in the early stages of GN mediated through inhibition of leukocyte infiltration and or adhesion30 and are proposed to act as endogenously produced proresolution agents in host defense and inflammation.31 As observed in intestinal enterocytes, expression of the LXA4 receptor was up-regulated in response to cytokines, furthermore addition of LXA4 or its stable, synthetic analogues inhibited interleukin-8 chemokine release, thereby modulating the initiation of inflammation.32 Previous data suggest that the anti-proliferative effects of LXA4 are mediated via intracellular mechanisms associated with modulation of growth factor receptor (PDGF and EGF) activation,4,6 and recruitment of specific SH-2 containing signaling molecules (Mitchell D, Gaffney A, Crean JK, Kinsella BT, Godson C, submitted). Here we demonstrate that LXA4 can counteract PDGF-induced gene expression. In agreement with other models in-vestigating LXA4-induced differential gene expression,18,33,34 we report that LXA4 modulated the expression of the NAB1 co-repressor, up-regulating expression by 0.3 SLRs. The stable synthetic analogue 15-epi-16-(para-fluorophenoxy)-lipoxin A (4)-methyl ester has been shown to exert remarkable renoprotection in a murine model of ischemia-reperfusion injury. Several of the genes differentially expressed in this model were also observed here, including interleukin-6, thrombospondin-1, metallothionein 1, transgelin, phosphoserine aminotransferase, and Enigma. LXA4 diminished the effects of PDGF-stimulated genes responsible for biological functions such as fibrosis, cell proliferation, immune response, lipid metabolism, and embryogenesis. Among the numerous genes identified as PDGF-responsive, those contributing to fibrosis-related changes in hMCs were clustered and examined further. We report that LXA4 modulates the PDGF-induced expression of several matrix-associated genes, including collagens, transforming growth factors, and fibronectin. Moreover, PDGF-increased expressions of matrix-associated proteins, thrombospondin, and fibronectin, were reduced by LXA4 to levels that were not significantly different to those of baseline. Such proteins contain multiple domains that bind proteoglycans, collagens, integrins, and cytokines, including TGF-β1 and are therefore important in the control of cell viability, mitogenesis, and motility. Fibronectin is present basally within the mesangial matrix functioning in supporting mesangial cell viability,35 increases in fibronectin have been demonstrated in fibrotic disease processes.36 TSP is transiently expressed in matrix during development and repair23,37 and has previously been shown to respond to PDGF, TGF-β1, and basic fibroblast growth factor,37 it is a known regulator of TGF-β1 activity.23,38 We further verify that levels of decorin, a small leucine-rich proteoglycan that acts as an endogenous inhibitor of TGF-β1,39 are decreased in response to PDGF and are recovered by LXA4 pretreatment. Overall, the up-regulation of both RNA and protein levels of the various fibrosis-associated genes, suggests an expansion of glomerular matrix and associated cytokines. The decreased expression of a negative regulator (decorin) and a corresponding increase in a positive regulator (thrombospondin) of TGF-β1 demonstrates that PDGF-induced profibrotic activity in hMCs may be associated with increases in TGF-β1. In contrast, LXA4 significantly reduced this PDGF-induced profibrotic activity observed in hMCs. These data further support the observed reduction in PDGF-induced secretion of TGF-β1 by LXA4 at 24, 48,6 and 72 hours. The diverse changes in matrix-associated proteins occurring throughout time, relate to initial injury and long-term damage sustained and the ultimate healing process thereafter. Secreted MMP-1 (collagenase I) levels were significantly enhanced in response to PDGF at 6, 18, and 24 hours, whereas LXA4 achieved diminution of the PDGF effect only at 6 and 18 hours. However, consistent with this MMP-1 RNA levels in response to PDGF were not significantly altered by LXA4 at 24 hours.
The observed dynamics of extracellular matrix synthesis and degradation are consistent with various models of glomerular and tubulointerstitial renal disease. Adhikary and colleagues40 examined various extracellular matrix components in anti-glomerular basement membrane nephritis, mRNA for fibronectin and collagens I and IV were elevated in glomeruli from day 15, throughout the disease course. TIMP-1 and TGF-β1 mRNA were also enhanced. A similar profile of extracellular matrix components and TGF-β1 was observed in the cortex, increasing more gradually from day 15 to day 29 as tubular damage progressed.40
The reduction in secreted levels of both MMP-1 and TGF-β1 in LXA4 pretreated cells may reflect reduced autokinase activity of the receptor tyrosine kinases (RTKs) and/or enhanced tyrosine phosphatase activity (Mitchell D, Gaffney A, Crean JK, Kinsella BT, Godson C, submitted). Significant evidence for the cross-talk between the LX G-protein-coupled receptor activated by LXA4 (ALXR) and the RTKs for both PDGF and EGF has been shown.4,6 In the context of LXA4 modulation of profibrotic changes, it is noteworthy that a role for the ALXR has recently been proposed in the anti-fibrotic treatment of a lung fibrosis model in mice.41 In these experiments LXA4 also significantly prevented enhanced proliferation of NIH3T3 fibroblasts and collagen expression by TGF-β1. Furthermore Sodin-Semrl and colleagues42 described a role for LXA4 in regulating human synovial fibroblast activation, levels of MMP-1 and MMP-3 were diminished in response to LXA4 treatment of stimulated fibroblasts.
The effects of LXA4 on PDGFR activation are relatively specific, being restricted to recruitment to precise phosphotyrosine residues (Mitchell D, Gaffney A, Crean JK, Kinsella BT, Godson C, submitted). In this regard it was of interest to investigate whether LXA4 might modulate transcription factors downstream of specific signal transduction processes. It has been reported that LXA4 can mediate NF-κB-induced gene expression in intestinal epithelial cells.43 Using MatInspector, many ubiquitous TF binding sequences were observed in the promoters of PDGF-induced genes implicated in both matrix-associated and proliferative gene regulation. Verrecchia and colleagues44 showed that inhibition of the SP-1 TF prevented the expression of extracellular matrix genes in dermal fibroblasts. Although SP-1 is a ubiquitous TF, it has also been shown to respond to several growth factors, MAPK and glucose.45 Posttranslational modification of SP-1 may have a significant role in TF activation. In our study we measured the binding of nuclear SP-1 from hMCs treated with PDGF and PDGF pretreated with LXA4, we found that SP-1 was significantly increased in response to PDGF. LXA4 consistently diminished this effect.
The translation of a relatively modest glomerular injury to devastating tubulointerstitial fibrosis is increasingly appreciated. Such fibrosis can reflect on a combination of EMT of resident epithelia or progenitor cells, infiltration of circulating or proliferation of resident fibroblasts.46,47 Alterations in the mediators present in the interstitium and in the glomerular filtrate contribute to the development of tubulointerstitial fibrosis.26 Tubulointerstitial fibrosis encompasses loss of epithelium polarity, adherens junctions, tight junctions, desmosomes, and cytokeratin intermediate filaments to rearrange their F-actin stress fibers and express filopodia and lamellopodia.48 Epithelial cells gain plasticity during the remodeling process, promoting healing or scarring as a response to injury.49 Induction of EMT is associated with the expression of cytokines and the proteolytic degradation of epithelial basement membrane.50 Matrix metalloproteinases or membrane assembly inhibitors dismantle the membrane, whereas local expression of TGF-β, EGF, IGF-II, or FGF-2 facilitates EMT.48,51,52 These cytokines contribute to elevated levels of matrix metalloproteinases and alter the cell proteome. Higgins and colleagues53 observed increases in the expression of collagen I and PAI-1, among others, in EMT using the in vivo model of unilateral ureteral obstruction and the in vitro model of stimulated murine proximal tubular (MCT) cultured cells. Signature markers for the disease were identified using oligonucleotide microarray.
We report here that PDGF-treated hMC supernatant caused a morphological change in renal tubular MCT cells, similar to that of TGF-β1 and EGF. Loss of epithelial tight junction marker E-cadherin and gain of mesenchymal actin cytoskeleton marker α-SMA in MCT cells treated with PDGF-stimulated hMC supernatant was observed. Interestingly, these effects were diminished by LXA4 pretreatment. LXA4 pretreatment could alter these effects perhaps by altering PDGF-induced receptor activation in the hMCs, changing the spectrum of soluble factors present in the ultrafiltrate. Furthermore, immunoblotting for E-cadherin and α-SMA under the same conditions confirmed these findings. Previously, we have reported the inhibition of PDGF and EGF mediated PKB/Akt phosphorylation by LXA4.6 This pathway may be involved in the release of cytokines or mediators from hMCs, which we observe to cause morphological changes in MCT cells, a role for this pathway in fibrosis is well established. Stimulation of fibroblasts with endothelin-1 promotes enhanced contractile phenotype or activation, this is prevented by blockade of the PI3K/Akt pathway.54 In agreement with this Vittal and colleagues55 observed that inhibition of PKB/Akt in bleomycin-induced lung fibrosis markedly reduced accumulation of α-smooth muscle actin-expressing myofibroblasts.
Supernatant from PDGF-stimulated hMCs stimulated an increase in RNA expression of VEGF, CTGF, and fibronectin. This effect was modulated by LXA4 pretreatment. To control for the possibility that residual PDGF in the media from pretreated hMCs might have induced profibrotic changes in the epithelial cells, these cells were treated with the PDGF-specific phosphotyrosine inhibitor, AG1296. However, in these cells changes in profibrotic gene expression persisted. Similarly, treatment of MCT cells with PDGF ligand did not induce profibrotic gene expression. Soluble factors released from hMCs in response to PDGF in our in vitro model may indicate the progression of renal disease seen in vivo.
Collectively these data demonstrate LXA4 as a potential anti-fibrotic agent, preventing growth factor-induced mesangial matrix production and the progression of renal disease, by alleviating the effect of hMC products on tubular cells. Further investigation into these bioactivities of LXA4 and its stable synthetic analogues will include examining effects on hMCs and tubular cells in an in vivo model of progressive renal disease.
Acknowledgments
We thank Dr. Hugh Brady for interest and advice.
Footnotes
Address reprint requests to Prof. Catherine Godson, Department of Medicine and Therapeutics, The Conway Institute for Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: catherine.godson@ucd.ie.
Supported by The Health Research Board Ireland, The Mater Foundation, The Wellcome Trust, The Government of Ireland Programe for Research in Third Level Institutes administered through the Higher Education Authority, and an Amgen Renal Research Bursary (to K.R.).
K.R. and B.M. contributed equally towards this manuscript.
Supplemental data (all Affymetrix data) are provided at www.ebi.ac.uk.
References
- Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- Floege J, Eng E, Young BA, Johnson RJ. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993;39:S47–S54. [PubMed] [Google Scholar]
- Abboud HE, Grandaliano G, Pinzani M, Knauss T, Pierce GF, Jaffer F. Actions of platelet-derived growth factor isoforms in mesangial cells. J Cell Physiol. 1994;158:140–150. doi: 10.1002/jcp.1041580118. [DOI] [PubMed] [Google Scholar]
- McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J. 2002;16:1817–1819. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- Johnson RRE, Floege J, Yoshimura A, Pritzl P, Alpers C, Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Rodgers K, Hanly J, McMahon B, Brady HR, Martin F, Godson C. Lipoxins inhibit Akt/PKB activation and cell cycle progression in human mesangial cells. Am J Pathol. 2004;164:937–946. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud HE. Role of platelet-derived growth factor in renal injury. Annu Rev Physiol. 1995;57:297–309. doi: 10.1146/annurev.ph.57.030195.001501. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Kelly DJ, McKay T, Chadban S, Hill PA, Cooper ME, Atkins RC, Nikolic-Paterson DJ. PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:1324–1332. doi: 10.1046/j.1523-1755.2001.0590041324.x. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, Kunter U, van Roeyen C, Dooley S, Janjic N, Ruckman J, Eitner F, Floege J. The effects of platelet-derived growth factor antagonism in experimental glomerulonephritis are independent of the transforming growth factor-beta system. J Am Soc Nephrol. 2002;13:658–667. doi: 10.1681/ASN.V133658. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. Scientific World J. 2002;2:169–204. doi: 10.1100/tsw.2002.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Bozza PT, Diaz BL, Cordeiro RS, Jose PJ, Martins MA, Serhan CN. Cutting edge: lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- McMahon B, Stenson C, McPhillips F, Fanning A, Brady HR, Godson C. Lipoxin A4 antagonizes the mitogenic effects of leukotriene D4 in human renal mesangial cells. Differential activation of MAP kinases through distinct receptors. J Biol Chem. 2000;275:27566–27575. doi: 10.1074/jbc.M001015200. [DOI] [PubMed] [Google Scholar]
- Mene P. Mesangial cell cultures. J Nephrol. 2001;14:198–203. [PubMed] [Google Scholar]
- Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Sadlier DM, Connolly SB, Kieran NE, Roxburgh S, Brazil DP, Kairaitis L, Wang Y, Harris DC, Doran P, Brady HR. Sequential extracellular matrix-focused and baited-global cluster analysis of serial transcriptomic profiles identifies candidate modulators of renal tubulointerstitial fibrosis in murine adriamycin induced nephropathy. J Biol Chem. 2004;279:29670–29680. doi: 10.1074/jbc.M313408200. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghici S, Khatri P, Martins RP, Ostermeier GC, Krawetz SA. Global functional profiling of gene expression. Genomics. 2003;81:98–104. doi: 10.1016/s0888-7543(02)00021-6. [DOI] [PubMed] [Google Scholar]
- Werner T. Target gene identification from expression array data by promoter analysis. Biomol Eng. 2001;17:87–94. doi: 10.1016/s1389-0344(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Harper JR, Spiro RC, Gaarde WA, Tamura RN, Pierschbacher MD, Noble NA, Stecker KK, Border WA. Role of transforming growth factor beta and decorin in controlling fibrosis. Methods Enzymol. 1994;245:241–254. doi: 10.1016/0076-6879(94)45014-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ. Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis. 1994;23:193–198. doi: 10.1016/s0272-6386(12)80971-1. [DOI] [PubMed] [Google Scholar]
- Ludewig D, Kosmehl H, Sommer M, Bohmer FD, Stein G. PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res. 2000;299:97–103. doi: 10.1007/s004419900118. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu FH, Devchand PR, Wada K, Serhan CN. Aspirin-triggered lipoxin A4 and lipoxin A4 up-regulate transcriptional corepressor NAB1 in human neutrophils. FASEB J. 2001;15:2736–2738. doi: 10.1096/fj.01-0576fje. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Hannan K, Burne MJ, Lappin DW, Doran P, Coleman P, Stenson C, Taylor CT, Daniels F, Godson C, Petasis NA, Rabb H, Brady HR. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, Makino H. Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int. 1998;54:1188–1196. doi: 10.1046/j.1523-1755.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Barnes JL, Mitchell RJ, Kanalas JJ, Barnes VL. Differential expression of thrombospondin and cellular fibronectin during remodeling in proliferative glomerulonephritis. J Histochem Cytochem. 1999;47:533–544. doi: 10.1177/002215549904700412. [DOI] [PubMed] [Google Scholar]
- Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ. Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and bFGF in vivo. Kidney Int. 1995;48:1846–1856. doi: 10.1038/ki.1995.483. [DOI] [PubMed] [Google Scholar]
- Daniel C, Takabatake Y, Mizui M, Isaka Y, Kawashi H, Rupprecht H, Imai E, Hugo C. Antisense oligonucleotides against thrombospondin-1 inhibit activation of TGF-beta in fibrotic renal disease in the rat in vivo. Am J Pathol. 2003;163:1185–1192. doi: 10.1016/s0002-9440(10)63478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijun W, Long C, Zhigang Z, Feng J, Muyi G. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol. 2005;78:17–24. doi: 10.1016/j.yexmp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Adhikary LP, Yamamoto T, Isome M, Nakano Y, Kawasaki K, Yaoita E, Kihara I. Expression profile of extracellular matrix and its regulatory proteins during the process of interstitial fibrosis after anti-glomerular basement membrane antibody-induced glomerular sclerosis in Sprague-Dawley rats. Pathol Int. 1999;49:716–725. doi: 10.1046/j.1440-1827.1999.00939.x. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kitasato H, Murakami Y, Hashimoto A, Endo H, Kondo H, Inoue M, Hayashi I. Down-regulation of lipoxin A4 receptor by thromboxane A2 signaling in RAW246.7 cells in vitro and bleomycin-induced lung fibrosis in vivo. Biomed Pharmacother. 2004;58:381–387. doi: 10.1016/j.biopha.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sodin-Semrl S, Spagnolo A, Barbaro B, Varga J, Fiore S. Lipoxin A4 counteracts synergistic activation of human fibroblast-like synoviocytes. Int J Immunopathol Pharmacol. 2004;17:15–25. doi: 10.1177/039463200401700103. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Rossert J, Mauviel A. Blocking sp-1 transcription factor broadly inhibits extracellular matrix gene expression in vitro and in vivo: implications for the treatment of tissue fibrosis. J Invest Dermatol. 2001;116:755–763. doi: 10.1046/j.1523-1747.2001.01326.x. [DOI] [PubMed] [Google Scholar]
- Goldberg HJ, Whiteside CI, Fantus IG. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-beta I and -delta. J Biol Chem. 2002;277:33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R, Wang S. Proteinuria and growth factors in the development of tubulointerstitial injury and scarring in kidney disease. Curr Opin Nephrol Hypertens. 2005;14:43–52. doi: 10.1097/00041552-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nahas AM. Plasticity of kidney cells: role in kidney remodeling and scarring. Kidney Int. 2003;64:1553–1563. doi: 10.1046/j.1523-1755.2003.00255.x. [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- Okada H, Inoue T, Suzuki H, Strutz F, Neilson EG. Epithelial-mesenchymal transformation of renal tubular epithelial cells in vitro and in vivo. Nephrol Dial Transplant. 2000;15(Suppl 6):44–46. doi: 10.1093/ndt/15.suppl_6.44. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Lappin DW, Kieran NE, Anders HJ, Watson RW, Strutz F, Schlondorff D, Haase VH, Fitzpatrick JM, Godson C, Brady HR. DNA oligonucleotide microarray technology identifies fisp-12 among other potential fibrogenic genes following murine unilateral ureteral obstruction (UUO): modulation during epithelial-mesenchymal transition. Kidney Int. 2003;64:2079–2091. doi: 10.1046/j.1523-1755.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]