Abstract
Diabetes impairs numerous aspects of tissue repair. Failure of wound angiogenesis is known to delay diabetic wound healing, whereas the importance of lymphangiogenesis for wound healing is unclear. We have examined whether overexpression of vascular endothelial growth factor (VEGF)-C via an adenoviral vector could improve the healing of full-thickness punch biopsy wounds in genetically diabetic (db/db) mice. We found that VEGF-C enhanced angiogenesis and lymphangiogenesis in the wound and significantly accelerated wound healing in comparison to the control wounds. VEGF-C also recruited inflammatory cells, some of which expressed VEGFR-3. On the other hand, when the function of endogenous VEGF-C/VEGF-D was blocked with a specific inhibitor, wound closure was delayed even further. These results suggest a function for VEGF-C in wound healing and demonstrate the therapeutic potential of VEGF-C in the treatment of diabetic wounds.
The wound healing process is a complex cascade that relies on several mechanisms for tissue repair including inflammation, granulation tissue formation, re-epithelialization, angiogenesis, and lymphangiogenesis.1 These processes are driven by growth factors secreted by inflammatory cells and other stromal cells in response to tissue injury. The clinical importance of blood perfusion in the healing tissue is evident in diseases that lead to compromised microcirculation, such as arterial and venous insufficiency, lymphedema, and diabetes. In these diseases, localized ischemia and edema inhibit the delivery of oxygen and nutrients to the tissues, impairing wound healing.
Insufficient blood perfusion coupled with impaired angiogenesis complicates tissue repair in diabetes. The potential use of therapeutic angiogenesis to improve wound healing has raised considerable interest. Vascular endothelial growth factors (VEGFs) are considered powerful therapeutic tools for proangiogenic and prolymphangiogenic therapy in many settings.2,3 The mammalian VEGF growth factor family consists of five members—VEGF, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor—representing key regulators of physiological and pathological vasculogenesis, angiogenesis, lymphangiogenesis, and vascular permeability.4 Their effects are mediated via tyrosine kinase receptors VEGFR-1, VEGFR-2, and VEGFR-3, which are predominantly expressed in vascular endothelial cells. VEGF binds to VEGFR-1 and VEGFR-2 and plays a crucial role in angiogenesis and tissue repair.5,6 VEGFR-2, which is expressed primarily in the blood vascular endothelium, is considered to be the main mediator of angiogenesis and vascular permeability.7 VEGF-C and VEGFR-3 are critical for the development and growth of the lymphatic vessels.8,9 VEGFR-3, the primary target receptor of VEGF-C, is expressed in the lymphatic endothelium, a few fenestrated endothelia,10 monocytes/macrophages,11,12 and a subpopulation of dendritic cells.13 However, at sites of active angiogenesis, such as tumors and chronic wounds, VEGFR-3 can be abnormally expressed in the blood vessel endothelium.14,15 Furthermore, the proteolytically processed mature form of VEGF-C (VEGF-CΔNΔC) can also activate VEGFR-2 in the blood vessel endothelium,16,17 induce angiogenesis, and increase vascular permeability in vivo.18,19 VEGF-C156S, a mutant form of VEGF-C, can only bind and activate VEGFR-3.20
Inflammation is required for normal wound healing, but this process is abnormal in diabetes. The diabetic wound is characterized by impaired inflammatory cell function, decreased secretion of cytokines/growth factors, and a prolonged inflammatory phase.21 The highly proteolytic microenvironment leads to a decreased activity of VEGF in diabetic wounds,22 while VEGF therapy accelerates diabetic wound healing.23,24 In contrast, the role of lymphangiogenic growth factors in tissue repair is unknown.
In this study, we have addressed the therapeutic effects of VEGF-C and the role of endogenous VEGF-C and VEGF-D during tissue repair. We have used adenoviral overexpression of VEGF-C, VEGF-C156S, VEGF, or soluble forms of VEGF receptors in full thickness punch biopsy wounds in genetically diabetic db/db mice.
Materials and Methods
Mice, Adenoviruses, and Growth Factor Expression
The Helsinki University Experimental Animal Care Committee and the District of Southern Finland approved all animal experiments. Ten-week-old obese diabetic BKS.Cg-m+/+ Lepr db/db mice (Taconic, Ejby, Denmark) and 8- to 10-week-old C57BLKS mice were used for the studies. Adenoviruses encoding human VEGF-C, VEGF-C156S, VEGF-A165, VEGF-B186, VEGFR-2-Ig, VEGFR-3-Ig, and β-galactosidase (LacZ) were constructed, and their protein expression was tested as described.3,25–27 In vivo protein production of the soluble receptor-immunoglobulin Fc fusion proteins was tested from serum with an enzyme-linked immunosorbent assay specific for human IgG Fc domain, as described.9
Experimental Wound Model
The mice were anesthetized with an intraperitoneal injection of xylazine (10 mg/kg) and ketamine (50 mg/kg). For analgesia, the mice received buprenorphine 0.1 to 0.5 mg/kg subcutaneously twice a day for 3 days after the operation. Circular paired punch biopsy skin wounds (3 to 5 mm) were made through the entire thickness of the skin on the dorsum of the mice after depilation with the Veet depilation creme containing thiglycollate (Reckit Benckiser, Mannheim, Germany). Adenoviruses (5 × 108 PFU) encoding human VEGF-C, VEGF-C156S, VEGF165, VEGF-B186 (as a control), VEGFR-2-Ig, VEGFR-3-Ig, or bacterial β-galactosidase were injected intradermally around the wound, and wounds were covered with a sterile transparent occlusive dressing (Tegaderm; 3M Health Care, St. Paul, MN), which was attached to the skin with cyano-acrylate glue and 5-0 nylon sutures. The dressing was removed on day 10 after wounding to enable visual analysis. Digital photographs of the dorsal wounds were taken every 3 days, and the wound area was calculated as a percentage of the original wound area. The mice were sacrificed on days 6, 18, or on the day of the wound closure. Four to six mice with paired wounds were analyzed for each time point. Another set of mice received 5 × 108 PFU of the different adenoviruses intra-dermally into the ear skin, and 2-mm circular penetrating punch biopsy wounds were made in the ear. These mice were sacrificed on day 6.
Morphological and Quantification Analysis of Vessels and Inflammatory Cells
The tissues were fixed with 1% paraformaldehyde perfusion through the left cardiac ventricle for 2 minutes and further incubated in 4% paraformaldehyde for 2 hours (the ear wounds) or overnight (the dorsal wounds). Deparaffinized sections were immunostained for the lymphatic vessel endothelial hyaluronan receptor-1 LYVE-1, or the platelet endothelial cell adhesion molecule-1 (PECAM-1) (BD Pharmingen).8 The samples were mounted with Aquamount (BHD) and visualized with a stereomicroscope (Leica). The blood and lymphatic vessels in the dorsal wounds were assessed in serial sections stained for LYVE-1 or PECAM-1. The numbers of PECAM-1-positive but LYVE-1-negative vessels or LYVE-1-positive vessels were counted from the wound edge (on day 6) and the granulation tissue (on day 18) using the same high-power magnification (×100 and ×200). The average value of at least eight different sections from four different mice in each study group was recorded in each study group. Five-μm frozen sections were stained with antibodies to mouse VEGFR-3 (R&D Systems) and the pan-hematopoietic marker CD45 (BD Pharmingen) or monocyte/macrophage marker 2 (MOMA2; Acris Antibodies) followed by incubation with donkey anti-goat Alexa594 and donkey anti-rat Alexa488 secondary antibodies (Molecular Probes). VEGF-C was detected using a rabbit antiserum to VEGF-C (no. 6) followed by incubation with Alexa594-conjugated secondary antibodies (Molecular Probes), whereas preimmune serum served as a negative control. Blood and lymphatic vessels in the ears were visualized by whole mount immunostaining as previously described,28 using fluorescent Alexa488, Alexa594 (Molecular Probes), or fluorescein isothiocyanate-conjugated secondary antibodies (Jackson Immunoresearch) for signal detection. Fluorescently labeled samples were mounted with Vectashield containing 4,6-diamidino-2-phenylindole (H-1200; Vector Laboratories), and analyzed with a compound fluorescent microscope (Zeiss 2; Carl Zeiss) or a confocal microscope (LSM 510; Zeiss) by using multichannel scanning in frame mode. Three-dimensional projections were digitally reconstructed from confocal z-stacks. CD45-, CD45/VEGFR-3-, MOMA2-, and MOMA2/VEGFR-3-positive cells were counted from 400× standard fluorescent micrographs. Co-localization of the signal was detected from 0.35-μm confocal optical sections using ×40 magnification.
Statistical Analysis
A two-tailed Student’s t-test was used to analyze differences between groups. All data are presented as mean ± 1 SD. A P value less than 0.05 was considered to be significant.
Results
Immunohistochemical staining of the unwounded skin of db/db mice and nondiabetic control mice for the pan-endothelial marker PECAM-1 and for the lymphatic endothelial marker LYVE-1 showed that the density of blood and lymphatic vessels was similar in both mouse lines (Supplementary Figure 1, see http://ajp.amjpathol.org). Adenoviral expression and growth factor protein production was tested in vitro and in vivo (data not shown). The expression of the transduced genes in the wounds localized to the forming granulation tissue next to the panniculus carnosum muscle that marked the original wound edge, as shown by X-gal staining for the LacZ adenovirus expression in the transfected wounds (Supplementary Figure 2A, see http://ajp.amjpathol.org). A strong signal for VEGF-C expression was also detected in a similar location on immunohistochemical staining of the AdVEGF-C-treated wounds (Supplementary Figure 2B, see http://ajp.amjpathol.org).
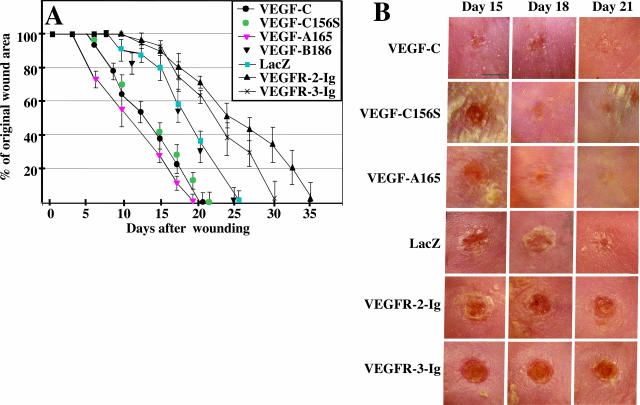
Figure 1.
VEGF-C accelerates the closure of full-thickness punch biopsy wounds. A: Diagram of the kinetics of wound closure in adenovirus-treated db/db mice. Each point represents the mean percentage of original wound size. Note that VEGF-C, VEGF-C156S, and VEGF significantly accelerate wound closure compared to VEGF-B and LacZ controls. Four to six mice were analyzed at each time point. B: Gross appearances of the wounds transfected with the different adenoviruses at the indicated time points. Note that VEGF-C-treated wounds have closed on day 21 after wounding whereas VEGFR-2-Ig- and VEGFR-3-Ig-treated wounds remain unepithelialized. Scale bar = 3 mm.
Figure 2.
VEGF-C induces angiogenic and lymphangiogenic sprouting at the wound edge. Whole mount staining of the wound edge (dashed line) on day 6 after wounding. The lymphatic marker LYVE-1 is shown in red, and the pan-endothelial marker PECAM-1 in green. A–C: Note dense blood and lymphatic capillary networks (A), lymphangiogenesis (B), and multiple angiogenic sprouts (arrows in A and C) in the VEGF-C-treated wounds. D–F: Less pronounced angiogenic (D, F) and lymphangiogenic (D, E) response in the VEGF-C156S-treated wounds. G and I: Note dense blood vessel network and numerous vessel spouts in VEGF-treated wounds. H: Some lymphatic vessel sprouts are also present. Note also the regressed vascular bed in the VEGFR-2-Ig-treated wound (M, O) and the absence of lymphatic sprouting and activation in the VEGFR-3-Ig-treated wound (Q) in contrast to the LacZ-treated (K, L) wounds. Capillary dilation is marked with arrowheads. Scale bars = 100 μm.
VEGF-C Accelerates Wound Closure
Paired full-thickness skin wounds in the back skin of the mice received adenoviral gene transfer vectors encoding VEGF-C, VEGF-C156S, VEGF-A165, soluble VEGFR-2-Ig, soluble VEGFR-3-Ig, and VEGF-B186 or β-galactosidase (LacZ) as controls. VEGF-C-, VEGF-C156S-, or VEGF-A-treated wounds showed significantly accelerated repair when compared to VEGF-B- or LacZ-treated wounds (Figure 1A). VEGF-C-treated wounds had a 20% reduced wound size by day 9; a similar reduction in the control group had occurred only by day 15. On day 18 only 22% of the original wound area was not epithelialized in the VEGF-C-treated wounds, whereas 58% of the LacZ control wounds remained unepithelialized. Wound closure was complete on average in 21 days in the VEGF-C-treated group, in 26 days in the LacZ group, and in 25 days in the VEGF-B-treated group. Wounds treated with viral vectors encoding the VEGFR-3-specific mutant growth factor VEGF-C156S displayed a nearly identical closure pattern when compared to the wild-type VEGF-C, and no statistical difference in wound closure was found at any time point. The differences in the mean wound sizes between the VEGF-C-, VEGF-C156S-, or VEGF-A- and control LacZ-treated (or VEGF-B) groups were statistically significant (P < 0.05) at all time points after day 8. However, wound closure was more advanced in the VEGF-A-treated wounds when compared to VEGF-C- or VEGF-C156S-treated wounds at the 7-day and 17-day time points (P < 0.05). At the time of wound closure, no statistical difference was observed between VEGF-C- and VEGF-A-treated wounds, whereas the closure of VEGF-C156S-treated wounds lagged behind that of the VEGF-A wounds (P = 0.01). When adenoviruses encoding soluble VEGFR-2-Ig and VEGFR-3-Ig were used to block the effects of endogenous VEGF-A (plus any proteolytically processed VEGF-C/D) and VEGF-C/D, respectively, both significantly delayed the wound closure (Figure 1, A and B). On average, wound closure took 35 days in the VEGFR-2-Ig group and 30 days in the VEGFR-3-Ig group. The differences in mean wound sizes between the VEGFR-2-Ig and LacZ groups were statistically significant after day 18, and between VEGFR-3-Ig and LacZ groups after day 21 (P < 0.05).
VEGF-C Enhances Angiogenesis and Lymphangiogenesis in the Wounds
Whole mount immunofluorescent staining for the blood vessel marker PECAM-1 and the lymphatic endothelial marker LYVE-1 were used to visualize vessels at the wound edge in the ear on day 6 after wounding (Figure 2). In the VEGF-C-treated wounds the PECAM-1-positive blood vessel network was dense, and angiogenic sprouting as well as capillary dilation were observed (Figure 2, A and C). Several PECAM-1-positive blood vessel sprouts were also seen in the VEGF-C156S-treated samples (Figure 2, D and F), whereas the strongest angiogenic response occurred in the VEGF-treated samples (Figure 2, G and I). LYVE-1 staining showed marked lymphangiogenic sprouting and enlargement of the lymphatic vessels in the VEGF-C- and VEGF-C156S-treated wounds (Figure 2, B and E). Some lymphatic vessel sprouts were also seen in the VEGF-treated samples (Figure 2H). In contrast, in wounds treated with the LacZ control virus, only a few blood and lymphatic vessel sprouts were observed (Figure 2, K and L; arrows). In the VEGFR-2-Ig-treated wounds, the blood vessels were thin, the capillaries were not dilated, neovascular sprouts were rare [Figure 2, M and O (arrow)], and lymphangiogenesis was not observed (Figure 2N). In the VEGFR-3-Ig-treated wounds the blood vessels were similar to the LacZ controls: capillary dilation and some angiogenic sprouts were seen (Figure 2, P and R), but the blood vessel network was less dense than in the LacZ-treated wounds (Figure 2, J and L). LYVE-1 staining indicated that VEGFR-3-Ig completely abolished even the background level of lymphatic activation and sprouting (Figure 2Q).
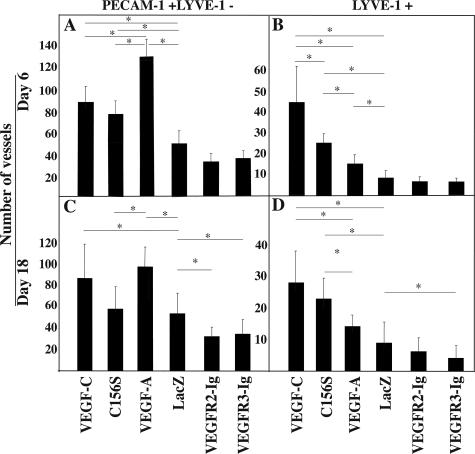
The number of PECAM-1-positive, LYVE-1-negative blood vessels and LYVE-1-positive lymphatic vessels was also recorded from the margins of the dorsal wounds (on day 6) or from the granulation tissue (on day 18) (Figure 3). VEGF-C induced a marked lymphangiogenic and angiogenic response at both time points (Figure 3). The angiogenic potential of VEGF-C was most evident at the wound edge on day 6, when the granulation tissue was just forming (compare the VEGF-C and LacZ columns in Figure 3A). VEGF-C induced angiogenesis was still present on day 18 in the mature granulation tissue (Figure 3C). Marked lymphangiogenesis was seen on day 6 in the edges of the VEGF-C-treated wounds, and lymphatic vessel proliferation persisted in the granulation tissue on day 18 (Figure 3, B and D). In comparison to LacZ-treated control wounds, the increase in blood and lymphatic vessel counts was statistically significant at both time points (Figure 3, A–D). In the VEGF-C156S-treated samples, a statistically significant increase in the number of blood vessels was found at day 6 but not at the day 18 time point (Figure 3, A and C), whereas a statistically significant lymphangiogenic effect was detected at both time points (Figure 3, B and D). The latter effect, however, was less prominent than in the VEGF-C group, especially at day 6 (Figure 3, B and D). VEGF induced the strongest angiogenic response at both time points (Figure 3, A and C); the difference in comparison to VEGF-C was statistically significant on day 6 but not on day 18 (Figure 3, A and C). VEGF also induced a statistically significant increase in the number of lymphatic vessels on day 6 but not on day 18 in comparison to the LacZ control samples (Figure 3, B and D).
Figure 3.
VEGF-C enhances angiogenesis and lymphangiogenesis in the wound bed. A–D: Quantitative analysis of PECAM-1+ and LYVE-1− blood vessels and LYVE-1+ lymphatic vessels in the dorsal wound sections on days 6 and 18. Bars represent mean values ± 1 SD (n = 8). P values less than 0.05 are marked by an asterisk.
Endogenous wound angiogenesis was evident on day 6 at the edges of the LacZ transduced control wounds (Figure 2L), whereas little if any endogenous wound-associated lymphangiogenesis was detected (Figure 2K), and thus the lymphatic vessel counts on day 6 were similar in the LacZ, VEGFR-2-Ig, and VEGFR-3-Ig groups (Figure 3B). However, by day 18 endogenous wound lymphangiogenesis was present in the granulation tissue also in the LacZ-treated wounds (data not shown). In the VEGFR-2-Ig-treated wounds, the number of PECAM-1-positive vessels appeared slightly decreased on day 6 (Figure 3A), but the difference was not statistically significant when compared to the LacZ group. On day 18, however, a significant decrease in the PECAM-1-positive blood vessels was evident in the VEGFR-2-Ig-treated wounds (Figure 3C). VEGFR-2-Ig had no statistically significant effects on the number of lymphatic vessels in the granulation tissue (Figure 3, D and H). VEGFR-3-Ig-treated wounds showed no statistically significant decrease of blood vessel density during the initial phase of wound healing (on day 6) when compared to the LacZ group (Figure 3A), but neovascularization of the granulation tissue was reduced on day 18 (Figure 3C). Also, lymphangiogenesis in the granulation tissue was impaired in the VEGFR-3-Ig-treated wounds on day 18 (Figure 3D).
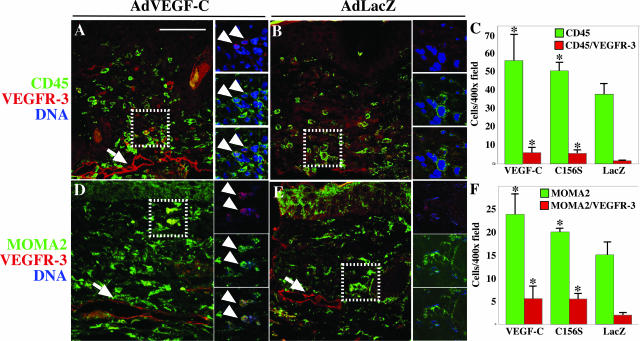
VEGF-C Recruits Hematopoietic Cells and Macrophages to the Wound Margin
To determine whether the beneficial effects of VEGF-C on wound closure, angiogenesis, and lymphangiogenesis occurred in parallel with increased recruitment of bone marrow-derived cells, we performed immunostaining for the pan-leukocyte marker CD45 that predominantly stains inflammatory cells in peripheral tissues. On day 6, in the VEGF-C- or VEGF-C156S-treated wounds more CD45-positive cells were found at the margin of the forming granulation tissue (Figure 4A and data not shown) when compared to LacZ-transduced wounds (Figure 4B). Some of the CD45-positive cells also expressed VEGFR-3 (Figure 4A, arrowheads), whereas fewer CD45/VEGFR-3-positive cells were found in the LacZ group (Figure 4B). Wounds treated with VEGF-C or VEGF-C156S contained 51 or 35%, more CD45-positive cells respectively, at the 6-day time point when compared to the LacZ group (VEGF-C versus LacZ, P = 0.045; VEGF-C156S versus LacZ, P = 0.043) (Figure 4C). The recruitment of VEGFR-3-positive leukocytes by VEGF-C and VEGF-C156S was increased by 2.5-fold (P = 0.019) and 2.3-fold (P = 0.022), respectively (Figure 4C).
Figure 4.
VEGF-C recruits hematopoietic cells and monocytes/macrophages to the wound margin. A and B: Immunofluorescent double staining of wound sections with antibodies to CD45 (green) and VEGFR-3 (red). D and E: Monocytes/macrophages stained with antibodies to MOMA2 (green), whereas VEGFR-3-positive cells are shown in red. Small panels on the right side of the large images are 0.35-μm confocal optical sections of the area highlighted with a dashed line in the large panel. Co-localization of the signal is indicated with arrowheads. C and F: Quantification of CD45 and CD45/VEGFR-3 (C) or MOMA2- and MOMA2/VEGFR-3-positive (F) cells from wound sections treated with the indicated viruses. P values less than 0.05 (comparison to the AdLacZ control) are marked by an asterisk. Bars represent mean values ± 1 SD (n ≥ 4). Scale bar = 100 μm.
Because VEGF-C has previously been shown to be a chemoattractant for monocytes/macrophages,11 we suspected that the majority of CD45-positive bone barrow-derived cells would be of the monocytic lineage. Indeed, increased numbers, 59% (P = 0.0013) and 33% (P = 0.036), of cells positive for the monocyte/macrophage marker MOMA2 were present in the VEGF-C- or VEGF-C156S-treated wounds (Figure 4D and data not shown), when compared to the LacZ-treated wounds (Figure 4E). Many of the MOMA2-positive cells also expressed VEGFR-3 (Figure 4D, arrowheads), suggesting that they are responsive to VEGF-C signals. Fewer VEGFR-3 expressing MOMA2-positive cells were found in the LacZ group (Figure 4E). The VEGFR-3-positive monocytes/macrophages were increased by 5.3-fold in the VEGF-C group (P = 0.017), and by 3.3-fold in the VEGF-C156S group (P = 0.0037) (Figure 4F).
Discussion
Our present study shows that VEGF-C and VEGF-C156S significantly accelerate diabetic wound healing by inducing persistent angiogenesis and lymphangiogenesis, as well as recruitment of inflammatory cells. VEGF-C and VEGF had similar effects on wound closure, whereas VEGF-B had no effect. Furthermore, blocking the function of endogenous VEGF-C/VEGF-D by a soluble receptor led to a severe delay in wound healing, suggesting that endogenous VEGF-C/VEGF-D are involved in diabetic wound healing.
Reduced expression and rapid proteolytic degradation of VEGF are considered partly responsible for poor wound healing in diabetes because these effects result in poor angiogenesis during granulation tissue formation.22,29 Although the function of VEGF in the diabetic wound is impaired, our present results show that VEGF-C also seems to play a role as an angiogenic growth factor in diabetic wound healing. This is consistent with the fact that the proteolytically processed mature form of VEGF-C is able to induce angiogenesis in vivo via VEGFR-2.16–19,30,31 Furthermore, VEGFR-3 expression is induced in the blood vessel endothelium of the granulation tissue.14,15 Our findings thus suggest that the diabetic wound microenvironment modulates the activities of VEGF-C, as recently suggested for VEGF.22 VEGF-B does not seem to induce significant angiogenesis or lymphangiogenesis in the tissues tested so far,32 which is probably why VEGF-B treatment did not improve wound healing.
Inflammation and accompanying fluid overload result in a lymphangiogenic response in some physiological and pathological conditions.33–35 In wound healing, the role of lymphangiogenic growth factors has not been established, although the expression of VEGF-C is known to be up-regulated during inflammation by proinflammatory cytokines and growth factors.35–37 Macrophages, being the prime source of several growth factors in wound healing, secrete VEGF-C and VEGF-D.12 These sources of lymphangiogenic factors are likely to contribute to the endogenous expression of VEGF-C during the wound healing process. According to our data, VEGF-C may contribute to wound healing by inducing the recruitment of inflammatory cells. Our results also suggest that, at least partly, the recruitment is induced directly via VEGFR-3, which is expressed in a subpopulation of macrophages.11,12 It also is conceivable that the increased vascular surface area, as well as increased vascular permeability, contributes to enhanced leukocyte transmigration in the VEGF-C-treated wounds. Macrophages isolated from diabetic db/db mice display a decrease in the secretion of nitric oxide, as well as various cytokines including tumor necrosis factor-α, interleukin-1β, and VEGF.38 Recruitment of additional macrophages to the wound may therefore compensate for the poor quality of macrophages by increasing their quantity, in this way enhancing perfusion (via nitric oxide) and angiogenesis (via VEGF), as well as the inflammatory response and endogenous VEGF-C production (via tumor necrosis factor-α and interleukin-1β).36,38 Macrophages recruited via the VEGFR-1-specific ligand placenta growth factor have been shown to be important for angiogenesis, vessel remodeling, and collateral formation in a rabbit hindlimb ischemia model,39 suggesting that macrophage recruitment could also contribute to angiogenesis in our model system. Interestingly, there were no statistically significant differences in the closure time of VEGF-C- and VEGF-C156S-treated wounds. The fact that at least on day 6 the VEGFR-3-specific VEGF-C156S enhanced angiogenesis in the wounds suggests that the angiogenic effect of VEGF-C and VEGF-C156S is partly mediated by monocytes/macrophages.
In addition to arterial inflow, venous and lymphatic outflow is also critical for wound healing. Venous insufficiency results in recurrent ulcers, and wound-associated lymphangiogenesis seems to fail in chronic wounds.14 Persistent local edema and delayed removal of local debris and inflammatory cells delay wound healing. Diabetic wounds remain chronically inflamed, leading to poor development of the granulation tissue and delayed wound closure.21,22 As we show here, VEGF-C accelerates diabetic wound healing by enhancing angiogenesis as well as lymphangiogenesis in the granulation tissue. Also VEGF possesses lymphangiogenic effects but these effects were not as pronounced as with VEGF-C or VEGF-C156S. Generation of new lymphatic vessels in the wound should facilitate the exit of excess fluid and leukocytes and concomitantly decrease the edema associated with the inflammatory response.34 Our results suggest a new biological role for VEGF-C in normal and diabetic wound healing and attest to the applicability of VEGF-C in the treatment of complicated diabetic wounds.
Supplementary Material
Acknowledgments
We thank Alun Parsons and Robert Paul for help in preparing the manuscript; Sanna Lampi, Mari Helanterä, and Tapio Tainola for excellent technical assistance; and the Biomedicum Helsinki Molecular Imaging Unit for technical help on imaging.
Footnotes
Address reprint requests to Dr. Kari Alitalo, Molecular/Cancer Biology Laboratory, Biomedicum Helsinki, P.O.B. 63 (Haartmaninkatu 8), University of Helsinki, 00014 Finland. E-mail: kari.alitalo@helsinki.fi.
Supported by the Academy of Finland (grants 202852 and 204312), the European Union (Lymphangiogenomics LSHG-CT-2004-503573), the National Institutes of Health (5 R01 HL075183-02), the Novo Nordisk Foundation, the Finnish Diabetes Foundation, the Maud Kuistila Foundation, and the Finnish Cultural Foundation.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med. 2002;196:719–730. doi: 10.1084/jem.20020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;(94):209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, Dana MR. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156:1499–1504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–497. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863–868. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J-H, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu J-S, Isner JM. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol. 1998;153:381–394. doi: 10.1016/S0002-9440(10)65582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. 1998;273:6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- Roth D, Piekarek M, Paulsson M, Christ H, Krieg T, Davidson JM, Eming SA. Plasmin modulates VEGF-A mediated angiogenesis during wound repair. Am J Pathol. 2006;168:670–684. doi: 10.2353/ajpath.2006.050372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–1277. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, Jackson DG, Yla-Herttuala S, Alitalo K. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623–629. doi: 10.1161/01.res.88.6.623. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Jackson DG, Ylä-Herttuala S, Jäättelä M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- Laitinen M, Makinen K, Manninen H, Matsi P, Kossila M, Agrawal RS, Pakkanen T, Luoma JS, Viita H, Hartikainen J, Alhava E, Laakso M, Yla-Herttuala S. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther. 1998;9:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361–373. doi: 10.1038/labinvest.3780244. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T, Alitalo K, Achen MG. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96:9671–9676. doi: 10.1073/pnas.96.17.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- Mouta C, Heroult M. Inflammatory triggers of lymphangiogenesis. Lymphat Res Biol. 2003;1:201–218. doi: 10.1089/153968503768330247. [DOI] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- Enholm B, Paavonen K, Ristimaki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II like db/db mice. Diabetes. 2000;49:145–148. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]
- Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.