Abstract
Volatile components present at spoilage of refrigerated chicken breasts were identified using high-vacuum-low-temperature distillation techniques followed by analysis with combined temperature-programmed gas chromatography and mass spectrometry. A comparison was made of the compounds detected from both irradiated and non-irradiated muscle stored at 2 and 10 degrees C under both aerobic and anaerobic conditions. Isolates were randomly selected from the spoiled poultry, identified, and evaluated for their ability to produce volatile spoilage noted when grown on radiation-sterilized chicken. Several isolates that produced off-odors on sterile chicken breasts were examined. Twenty-two compounds were associated with spoilage. Some of the compounds found on both irradiated and unirradiated samples were considered to play only a minor role in the spoilage aroma or were present in low concentrations, since the aroma of spoiled irradiated chicken lacked the harsh odor notes typical of spoiled unirradiated chicken. Fifteen of the 22 compounds were considered to be unique to unirradiated, aerobically spoiled samples. Nine of these compounds, hydrogen sulfide, methyl mercaptan, dimethyl sulfide, dimethyl disulfide, methyl acetate, ethyl acetate, heptadiene, methanol, and ethanol, were found on chicken spoiled at both 2 and 10 degrees C. xylene, benzaldehyde, and 2,3-dithiahexane were detected only in samples stored at 2 degrees C and methyl thiolacetate, 2-butanone, and ethyl propionate were associated with 10 degrees C spoilage. Fifty-eight isolates randomly selected from fresh, radiation-pasteurized, and unirradiated spoiled poultry were classified taxonomically, and 10 of them, which produced spoilage odors on sterilized chicken breasts, were selected for subsequent analysis of their volatiles. Isolates identified as Pseudomonas putrefaciens and Pseudomonas species that were members of groups I and II of Shewan's classification, as well as Flavobacterium and oxidative Moraxella, produced a number of the compounds found in the aroma of spoiled chicken. A total of 17 compounds were identified. Whereas no isolate produced all of the aroma compounds found in the aroma of spoiled chicken, together they did produce the nine found in unirradiated samples spoiled at either 2 or 10 degrees C, as well as methyl thiolacetate and xylene. Six compounds were present in the volatiles produced by the isolates but were absent in the volatiles identified from spoiled chicken. These were hydrogen cyanide, methyl isopropyl sulfide, 2-propane thiol, methyl propionate, ethyl benzene, and an unidentified compound.
Full text
PDF

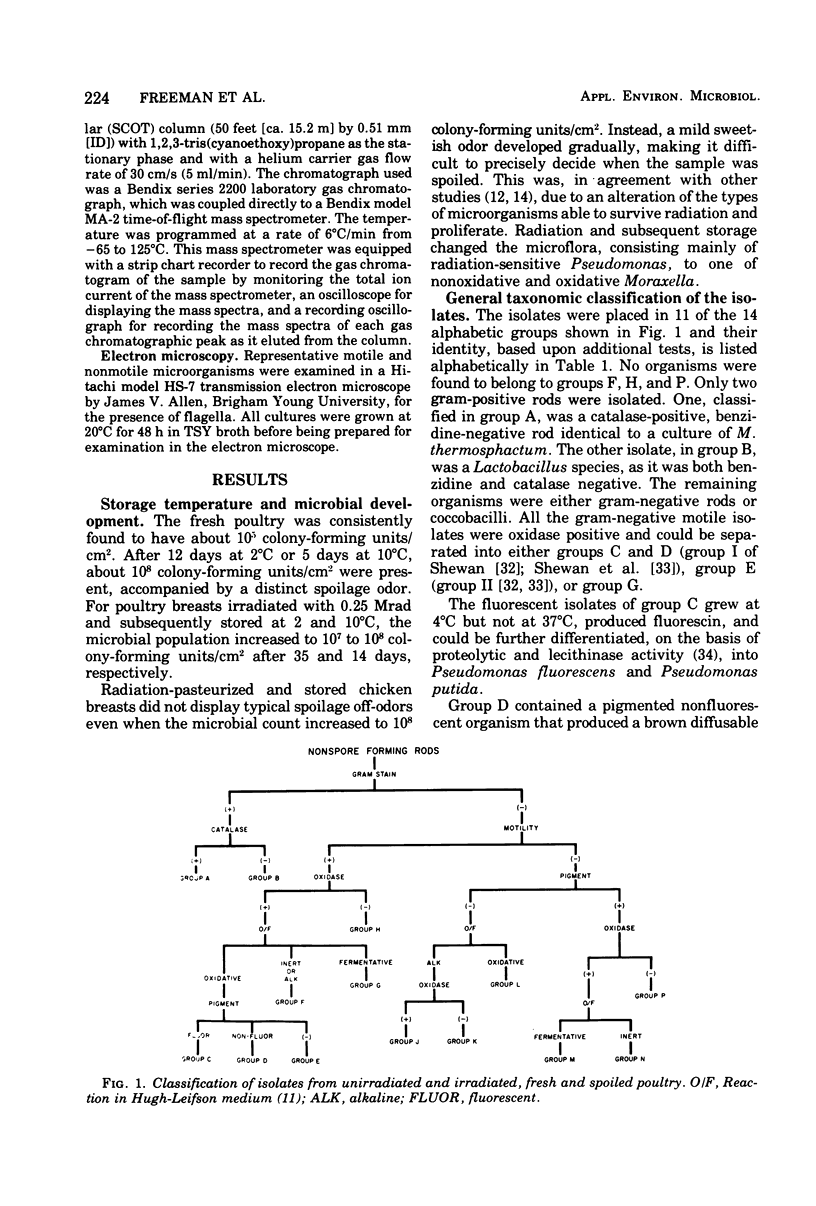
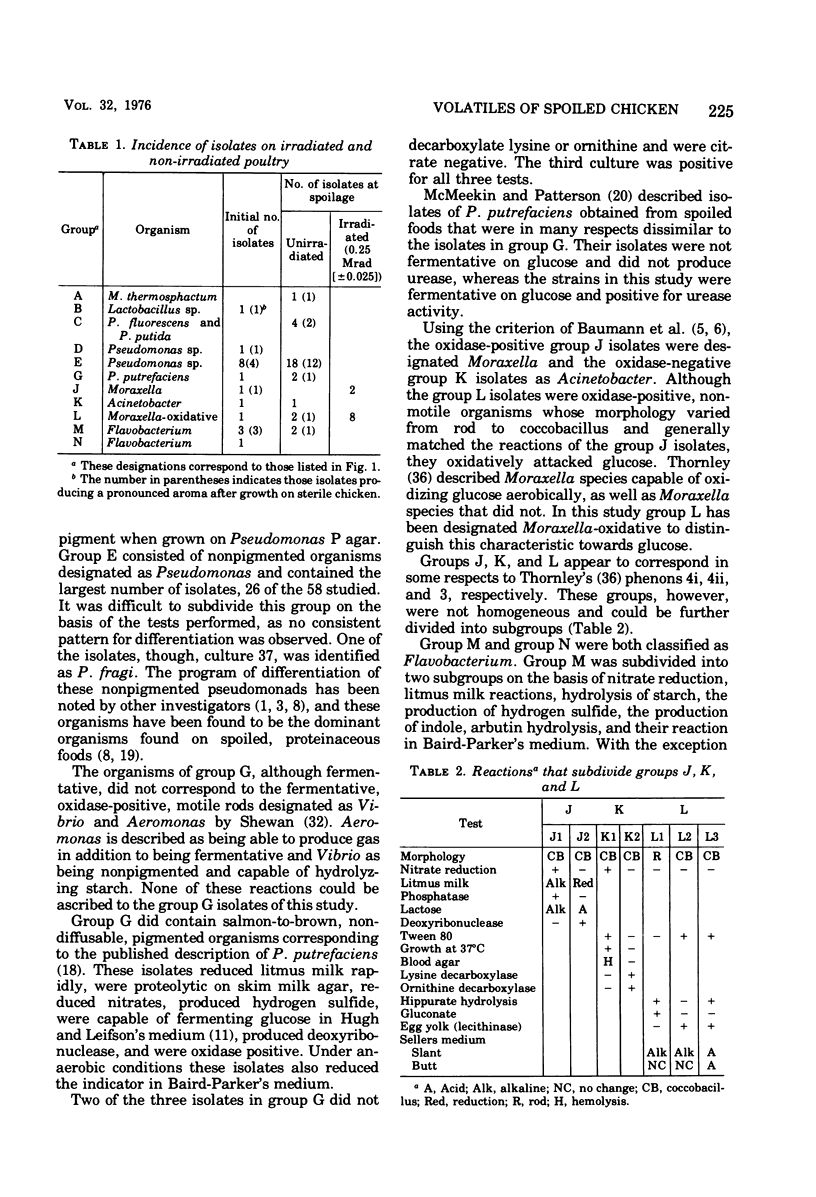


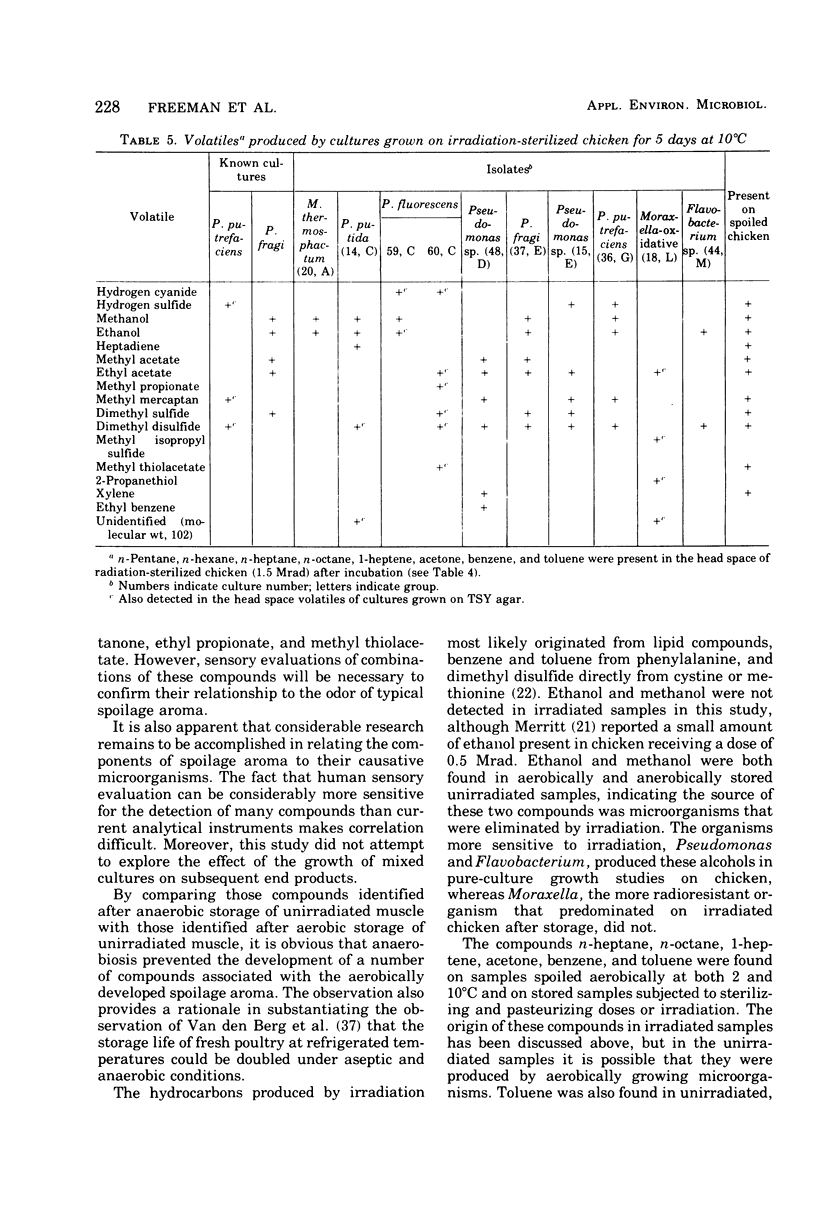



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Impey C. S. Psychrophilic spoilage bacteria of poultry. J Appl Bacteriol. 1968 Mar;31(1):97–107. doi: 10.1111/j.1365-2672.1968.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968 Jan;95(1):58–73. doi: 10.1128/jb.95.1.58-73.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Nawar W. W., Levin R. E. Identification of major high-boiling volatile compounds produced during refrigerated storage of haddock fillets. Appl Microbiol. 1974 Oct;28(4):679–680. doi: 10.1128/am.28.4.679-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. R., Angelini P., Silverman G. J., Merritt C., Jr Production of hydrogen cyanide by Pseudomonas fluorescens. Appl Microbiol. 1975 Apr;29(4):560–561. doi: 10.1128/am.29.4.560-561.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert R. A., Hendrie M. S., Gibson D. M., Shewan J. M. Symposium on microbial changes in foods. Bacteria active in the spoilage of certain sea foods. J Appl Bacteriol. 1971 Mar;34(1):41–50. doi: 10.1111/j.1365-2672.1971.tb02267.x. [DOI] [PubMed] [Google Scholar]
- INGRAM M., THORNLEY M. J. Changes in spoilage pattern of chicken meat as a result of irradiation. Int J Appl Radiat Isot. 1959 Oct;6:122–128. doi: 10.1016/0020-708x(59)90104-8. [DOI] [PubMed] [Google Scholar]
- Idziak E. S., Incze K. Radiation treatment of foods. I. Radurization of fresh eviscerated poultry. Appl Microbiol. 1968 Jul;16(7):1061–1066. doi: 10.1128/am.16.7.1061-1066.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram M., Dainty R. H. Symposium on microbial changes in foods. Changes caused by microbes in spoilage of meats. J Appl Bacteriol. 1971 Mar;34(1):21–39. doi: 10.1111/j.1365-2672.1971.tb02266.x. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Lea C. H., Parr L. J., Jackson H. F. Chemical and organoleptic changes in poultry meat resulting from the growth of psychrophylic spoilage bacteria at 1 degree C. 3. Glutamine, glutathione, tyrosine, ammonia, lactic acid, creatine, carbohydrate, haem pigment and hydrogen sulphide. Br Poult Sci. 1969 Jul;10(3):229–238. doi: 10.1080/00071666908415763. [DOI] [PubMed] [Google Scholar]
- Levin R. E. Detection and incidence of specific species of spoilage bacteria on fish. I. Methodology. Appl Microbiol. 1968 Nov;16(11):1734–1737. doi: 10.1128/am.16.11.1734-1737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeekin T. A., Patterson J. T. Characterization of hydrogen sulfide-producing bacteria isolated from meat and poultry plants. Appl Microbiol. 1975 Feb;29(2):165–169. doi: 10.1128/am.29.2.165-169.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeekin T. A. Spoilage association of chicken breast muscle. Appl Microbiol. 1975 Jan;29(1):44–47. doi: 10.1128/am.29.1.44-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., 3rd, Scanlan R. A., Lee J. S., Libbey L. M. Identification of the volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas fragi. Appl Microbiol. 1973 Jun;25(6):952–955. doi: 10.1128/am.25.6.952-955.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., 3rd, Scanlan R. A., Lee J. S., Libbey L. M., Morgan M. E. Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas perolens. Appl Microbiol. 1973 Feb;25(2):257–261. doi: 10.1128/am.25.2.257-261.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., 3rd, Scanlan R. A., Lee J. S., Libbey L. M. Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas putrefaciens, Pseudomonas fluorescens, and an Achromobacter species. Appl Microbiol. 1973 Jul;26(1):18–21. doi: 10.1128/am.26.1.18-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol D. J., Shaw M. K., Ledward D. A. Hydrogen sulfide production by bacteria and sulfmyoglobin formation in prepacked chilled beef. Appl Microbiol. 1970 Jun;19(6):937–939. doi: 10.1128/am.19.6.937-939.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron A. P., Dockstader W. B. Selective medium for hydrogen sulfide production by salmonellae. Appl Microbiol. 1972 Jun;23(6):1107–1112. doi: 10.1128/am.23.6.1107-1112.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. C., Bills D. D., Lindsay R. C. Ester production by Pseudomonas fragi. II. Factors influencing ester levels in milk cultures. Appl Microbiol. 1969 Jun;17(6):779–782. doi: 10.1128/am.17.6.779-782.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Thornley M. J. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 1967 Nov;49(2):211–257. doi: 10.1099/00221287-49-2-211. [DOI] [PubMed] [Google Scholar]


