Abstract
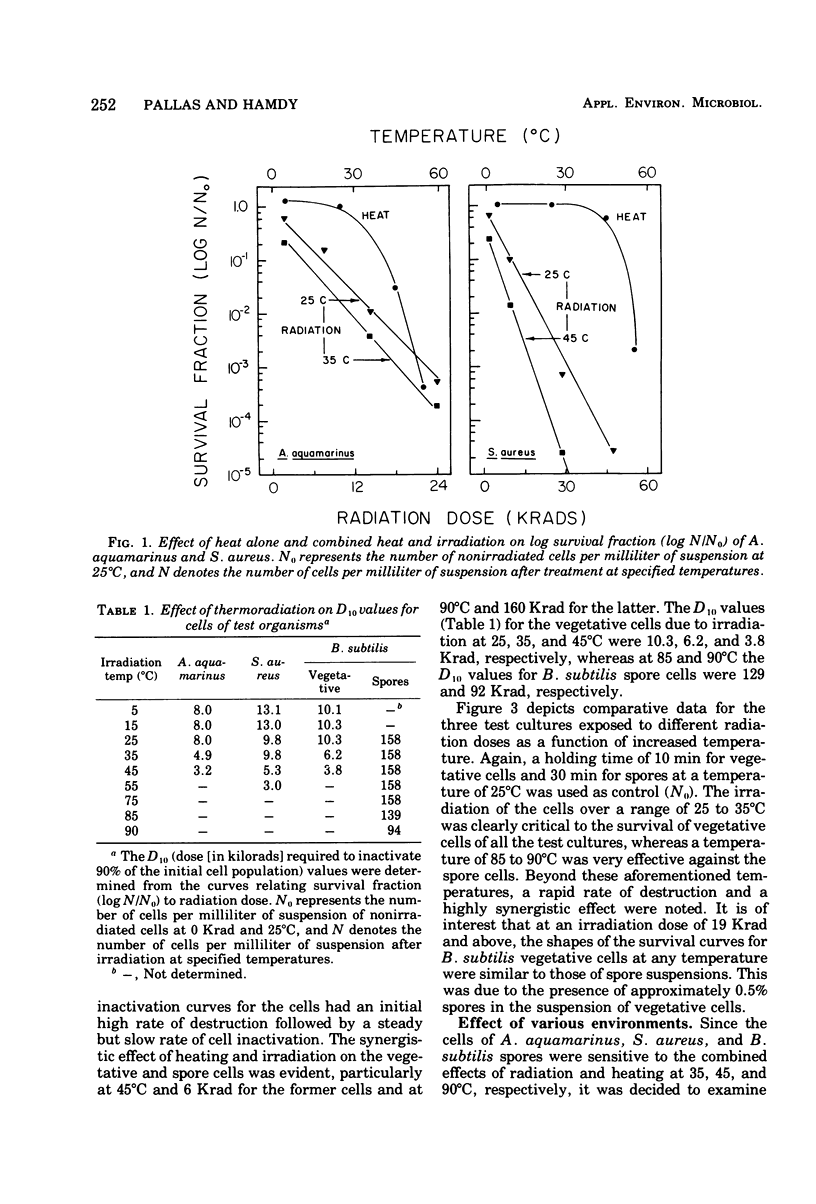
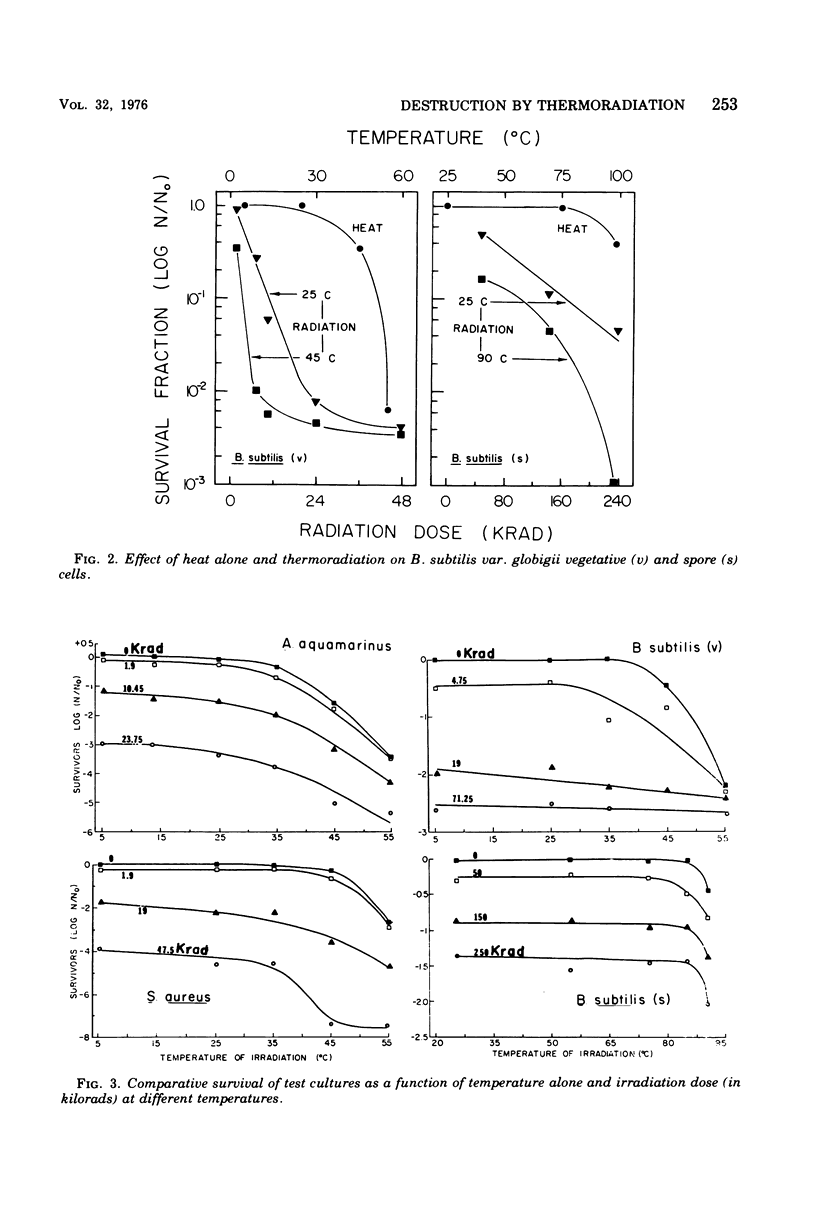
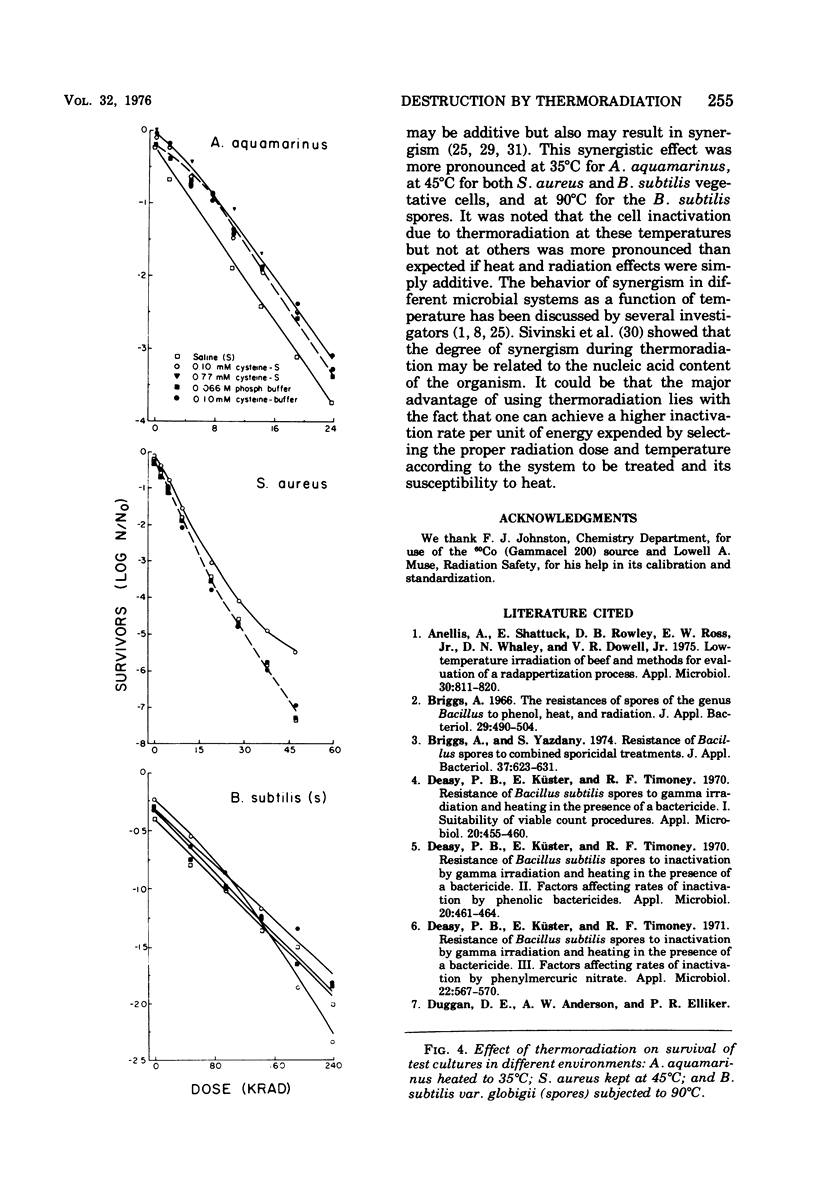
A 60Co source was used to determine the effects of thermoradiation on Achromobacter aquamarinus, Staphylococcus aureus, and vegetative and spore cells of Bacillus subtilis var. globigii. The rate of inactivation of these cultures, except vegetative-cell populations of B. subtilis, was exponential and in direct proportion to temperature. The D10 (dose that inactivates 90% of the microbial population) value for A. aquamarinus was 8.0 Krad at 25 degrees C and 4.9 Krad at 35 degrees C. For S. aureus, D10 was 9.8 and 5.3 Krad at 35 and 45 degrees C, respectively. Vegetative cells of B. subtilis demonstrated a rapid initial inactivation followed by a steady but decreased exponential rate. The D10 at 25 degrees C was 10.3 Krad, but at 35 and 45 degrees C this value was 6.2 and 3.8 Krad, respectively. Between 0 and 95 Krad, survival curves for B. subtilis spores at 75 degrees C showed slight inactivation, increasing in rat at and above 85 degrees C. The D10 values for spores at 85 and 90 degrees C were 129 and 92 Krad, respectively. Significant synergism between heat and irradiation was noted at 35 degrees C for A. aquamarinus and 45 degrees C for S. aureus. The presence of 0.1 mM cysteine in suspending media afforded protection to both cultures at these critical temperatures. On the other hand, cysteine sensitized B. subtilis spores at radiation doses greater than 100 Krad. The combined effect of heat and irradiation was more destructive to bacteria than either method alone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anellis A., Shattuck E., Rowley D. B., Ross E. W., Jr, Whaley D. N., Dowell V. R., Jr Low-temperature irradiation of beef and methods of evaluation of radappertization process. Appl Microbiol. 1975 Nov;30(5):811–820. doi: 10.1128/am.30.5.811-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs A. The resistances of spores of the genus Bacillus to phenol, heat and radiation. J Appl Bacteriol. 1966 Dec;29(3):490–504. doi: 10.1111/j.1365-2672.1966.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Briggs A., Yazdany S. Resistance of Bacillus spores to combined sporicidal treatments. J Appl Bacteriol. 1974 Dec;37(4):623–631. doi: 10.1111/j.1365-2672.1974.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Deasy P. B., Küster E., Timoney R. F. Resistance of Bacillus subtilis spores to inactivation by gamma irradiation and heating in the presence of a bactericide. 3. Factors affecting rates of inactivation by phenylmercuric nitrate. Appl Microbiol. 1971 Oct;22(4):567–570. doi: 10.1128/am.22.4.567-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy P. B., Küster E., Timoney R. F. Resistance of Bacillus subtilis spores to inactivation by gamma irradiation and heating in the presence of a bactericide. I. Suitability of viable count procedures. Appl Microbiol. 1970 Sep;20(3):455–460. doi: 10.1128/am.20.3.455-460.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy P. B., Küster E., Timoney R. F. Resistance of Bacillus subtilis spores to inactivation by gamma irradiation and heating in the presence of a bactericide. II. Factors affecting rates of inactivation by phenolic bactericides. Appl Microbiol. 1970 Sep;20(3):461–464. doi: 10.1128/am.20.3.461-464.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg C. Inactivation of dried bacteria and bacterial spores by means of gamma irradiation at high temperatures. Appl Microbiol. 1974 May;27(5):830–833. doi: 10.1128/am.27.5.830-833.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecz N., Walker A. A., Anellis A., Berkowitz D. Effect of irradiation temperature in the range--196 to 95C on the resistance of spores of Clostridium botulinum 33A in cooked beef. Can J Microbiol. 1971 Feb;17(2):135–142. doi: 10.1139/m71-024. [DOI] [PubMed] [Google Scholar]
- Hamdy M. K., Noaman M., Caster W. O. Effects of cysteine and 4-amino-1-naphthol on solutions of lysosomal enzymes exposed to high doses of radiation. Radiat Res. 1969 Apr;38(1):214–222. [PubMed] [Google Scholar]
- Hurst A., Hughes A., Duckworth M., Baddiley J. Loss of D-alanine during sublethal heating of Staphylococcus aureus S6 and magnesium binding during repair. J Gen Microbiol. 1975 Aug;89(2):277–284. doi: 10.1099/00221287-89-2-277. [DOI] [PubMed] [Google Scholar]
- KEMPE L. L. Combined effects on heat and radiation in food sterilization. Appl Microbiol. 1955 Nov;3(6):346–352. doi: 10.1128/am.3.6.346-352.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. F., Alur M. D., Kumta U. S. Radiation sensitivity of fish microflora. Indian J Exp Biol. 1971 Jan;9(1):45–47. [PubMed] [Google Scholar]
- Petkau A., Chelack W. S. Radioprotection of Acholeplasma laidlawii B by cysteine. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Apr;25(4):321–328. doi: 10.1080/09553007414550391. [DOI] [PubMed] [Google Scholar]
- Powers E. L., Richmond R. C., Simic M. OH radicals in radiation sensitization. Nat New Biol. 1972 Aug 30;238(87):260–261. doi: 10.1038/newbio238260a0. [DOI] [PubMed] [Google Scholar]
- Powers E. M. Method for obtaining free bacterial spores of Bacillus subtilis var. niger. Appl Microbiol. 1968 Jan;16(1):180–181. doi: 10.1128/am.16.1.180-181.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdie J. W., Ebert M., Tallentire A. Increased response of anoxic Bacillus megaterium spores to radiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Nov;26(5):435–443. doi: 10.1080/09553007414551461. [DOI] [PubMed] [Google Scholar]
- Reynolds M. C., Lindell K. F., David T. J., Favero M. S., Bond W. W. Thermoradiation inactivation of naturally occurring bacterial spores in soil. Appl Microbiol. 1974 Sep;28(3):406–410. doi: 10.1128/am.28.3.406-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R. C., Powers E. L. Modification of radiation sensitivity of bacterial spores by silver salts. Radiat Res. 1974 Jun;58(3):470–480. [PubMed] [Google Scholar]
- Richmond R. C., Simic M., Powers E. L. Radiation sensitivity of Bacillus megaterium spores in the presence of Co(III)complexes. Radiat Res. 1975 Jul;63(1):140–148. [PubMed] [Google Scholar]
- Rosenthal L. J., Iandolo J. J. Thermally induced intracellular alteration of ribosomal ribonucleic acid. J Bacteriol. 1970 Sep;103(3):833–835. doi: 10.1128/jb.103.3.833-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallentire A., Jones A. B. Radiosensitization of bacterial spores by potassium permanganate. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Oct;24(4):345–354. doi: 10.1080/09553007314551201. [DOI] [PubMed] [Google Scholar]


