Abstract
The p53 protein suppresses tumorigenesis by initiating cellular functions such as cell cycle arrest and apoptosis in response to DNA damage. A p53 mutant, p53R172P, which is deficient for apoptosis but retains a partial cell cycle arrest function, delays tumor onset in mice. Remarkably, lymphomas arising in Trp53515C/515C mice (encoding p53R172P) retain stable genomes. Given the dominant role of p21 in p53 cell cycle control, we crossed Trp53515C/515C mice onto a p21-null background to determine whether p21 was required for maintaining chromosomal stability and delaying tumor onset. Loss of p21 completely abolished the cell cycle arrest function of p53R172P and accelerated tumor onset in Trp53515C/515C mice. Cytogenetic examination of Trp53515C/515C p21−/− sarcomas and lymphomas revealed aneuploidy and chromosomal aberrations that were absent in Trp53515C/515C malignancies. Thus, p21 coupled p53-dependent checkpoint control and preservation of chromosomal stability, and cooperated with apoptosis in suppressing tumor onset in mice.
Keywords: apoptosis, chromosomal instability, p53, tumorigenesis, mouse model
The role of p53 in tumor suppression is well established. Transcriptional activation of a large repertoire of target genes implicates p53 in tumor-preventive functions, such as cell cycle control, apoptosis, and maintenance of chromosomal stability. Control of the cell cycle by p53 is primarily accomplished by activation of the cyclin-dependent kinase inhibitor, p21, resulting in a G1 arrest after DNA damage (1, 2). Loss of p21 severely compromises the G1 checkpoint control by p53 (3–5). However, p21−/− mice are resistant to early onset tumorigenesis (3, 6–8). Similarly, deletion of Gadd45, a p53 target gene involved in the G2/M progression, causes centrosome amplification and chromosomal instability (CIN) but does not predispose mice to tumorigenesis (9). These findings reveal the importance of proper cell cycle control in maintaining genomic integrity and suggest that cell cycle control plays a redundant role with other p53 functions in suppressing tumorigenesis.
Deletion of the p53 apoptotic targets, Noxa, Puma, and Bax, in mice also has demonstrated their dispensable role in preventing early tumor onset (10–13). To better address the role of p53-dependent cell cycle control and apoptosis in tumor suppression, we exploited the properties of a rare apoptosis-deficient human p53 Arg-to-Pro mutant, p53R175P, which retains a cell cycle arrest function (14, 15). Trp53515C/515C mice (encoding p53R172P, the corresponding murine mutant) are deficient for apoptosis but retain a partial cell cycle arrest function and have a prolonged survival compared with Trp53−/− mice (16). Importantly, Trp53515C/515C lymphomas that develop late have stable genomes unlike Trp53−/− malignancies, which are characterized by CIN (16, 17). These data demonstrate that p53-dependent apoptosis is not solely responsible for preventing tumor onset and suggest that p53 cell cycle control may suppress tumorigenesis by preservation of genomic integrity.
Given the dominant role of p21 in p53 cell cycle control, we generated double mutant mice, Trp53515C/515Cp21−/−, to test whether loss of proper cell cycle control via p21 resulted in CIN, enhancing tumor onset in Trp53515C/515C mice. Here, we demonstrate that p21 cell cycle control cooperates with the apoptotic pathway for effective tumor suppression by p53. Our results explain the failure of mouse models with deletion of individual p53 target genes to recapitulate a Trp53-null tumor phenotype (18). Thus, combination therapies targeting p53 cell cycle and apoptotic pathways are crucial for tumor suppression.
Results
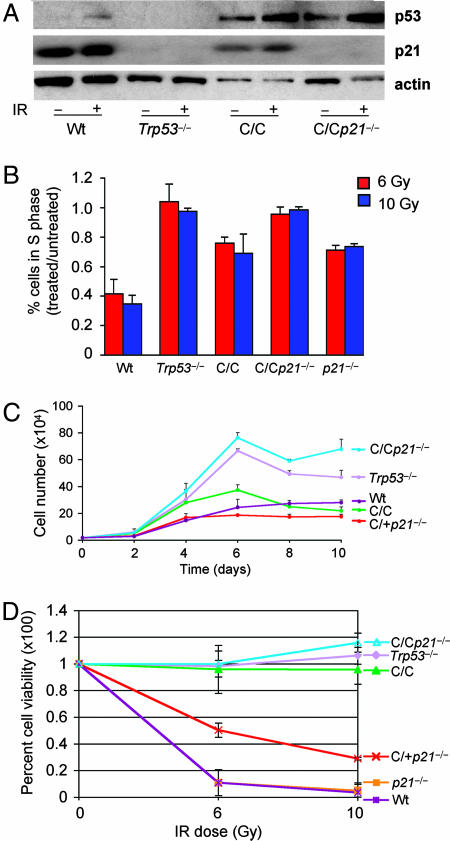
In response to DNA damage, p21 activation by p53 is essential in mediating a cell cycle arrest (3–5). To assay p53R172P induction of p21, Trp53515C/515C mouse embryo fibroblasts (MEFs) were exposed to DNA damage, and p21 levels were examined by immunoblot analysis. Like wild-type p53, p53R172P was stabilized after γ-radiation and induced the expression of p21 (Fig. 1A). Under the same conditions, p21 protein was not detected in Trp53−/− or Trp53515C/515Cp21−/− cells. We next addressed the importance of p21 in response to p53R172P by examining the cell cycle progression of Trp53515C/515Cp21−/− and Trp53515C/515C MEFs. Subconfluent cultures of early passage MEFs were treated with γ-radiation then labeled with BrdU to determine the proportion of cells in S phase. Trp53515C/515C and p21−/− MEFs retained a partial p53-dependent cell cycle arrest, as evidenced by a reduction in the ratio of cells in S phase after irradiation compared with Trp53−/− cells (Fig. 1B). In contrast, the number of Trp53−/− and Trp53515C/515Cp21−/− MEFs in S phase was similar after treatment, indicating the absence of a cell cycle arrest in these cells. These findings indicate that p21 entirely mediated the cell cycle checkpoint control by p53R172P in response to γ-radiation.
Fig. 1.
Trp53515C/515C p21−/− cells are deficient for apoptosis and cell cycle arrest. (A) Immunoblot analysis of p53 and p21 in MEFs of the indicated genotypes before and after treatment with 6 Gy of γ-radiation. Actin was used as a loading control. (B) Cell cycle progression was assayed by determining the ratio of cells in S phase from irradiated (6 Gy) to nonirradiated MEFs with different genotypes: wild-type (Wt), Trp53−/−, Trp53515C/515C (C/C), Trp53515C/515Cp21−/− (C/Cp21−/−), and p21−/−. (C) Equal numbers of MEFs of different genotypes at passage 2 were plated in triplicate and counted at the indicated times. Similar results were obtained from at least two independently derived MEF lines. (D) Apoptosis in mouse thymocytes after treatment with γ-radiation was measured by labeling cells with Annexin-V. Depicted are average values determined from at least three mice of each genotype with SE shown.
To further explore the importance of p21 to cell cycle control in response to p53R172P, we analyzed the growth rates and saturation densities of early passage MEFs. At day 4 of culture, Trp53515C/515Cp21−/− and Trp53−/− cells continued proliferating and attained a higher saturation density than Trp53515C/515C, wild-type, or Trp53515C/+p21−/− cells (Fig. 1C). Thus, p21 loss delayed the ability of p53R172P to arrest cell proliferation upon contact inhibition, resembling the Trp53-null phenotype. Taken together these data demonstrate the importance of p21 in mediating a p53-dependent cell cycle arrest.
Murine thymocytes undergo p53-dependent apoptosis upon exposure to γ-radiation (19). By using this methodology, we have previously shown that p53R172P is completely deficient for apoptosis in vivo (16). In further characterization of Trp53515C/515Cp21−/− mice, freshly isolated thymocytes were treated with γ-radiation and stained with Annexin-V, a marker for apoptosis, to determine cell viability. Trp53515C/515C, Trp53515C/515Cp21−/−, and Trp53−/− thymocytes remained viable after treatment with 6 and 10 Gy of γ-radiation (Fig. 1D). In contrast, wild-type and p21−/− cells displayed abundant cell death, whereas Trp53515C/+p21−/− cells displayed an intermediate viability. Thus, in response to γ-radiation, Trp53515C/515Cp21−/− cells, like Trp53−/− cells, were completely deficient for apoptosis and cell cycle arrest functions.
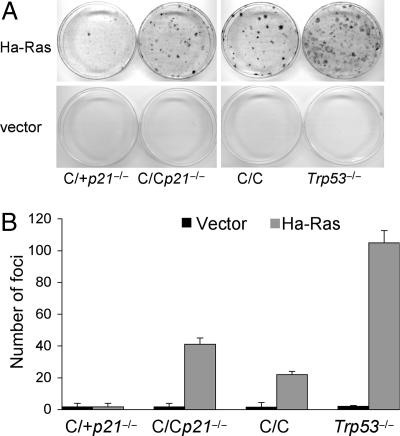
Trp53-deficient mouse fibroblasts bypass Ras-induced replicative senescence and undergo transformation (20). To begin to address the importance of p21 in tumor suppression in the absence of p53-dependent apoptosis, we assayed the focus-forming potential of early passage Trp53515C/515C, Trp53515C/515Cp21−/−, Trp53515C/+p21−/−, and Trp53−/− MEFs infected with a retroviral vector encoding an Ha-RasV12 cDNA. Trp53515C/515C MEFs exhibited a transformation potential in response to activated Ras, forming ≈20% the number of foci as Trp53−/− cells (Fig. 2). Loss of p21 enhanced transformation by oncogenic Ras as Trp53515C/515Cp21−/− cells formed ≈40% the number of foci as Trp53−/− cells. In contrast, few foci were formed by Trp53515C/+p21−/− cells. Thus, the p53R172P mutant exhibited transformation potential in combination with oncogenic Ras that was enhanced by loss of p21. Yet, loss of p21 alone in a wild-type p53 background did not affect transformation.
Fig. 2.
Loss of p21 enhances transformation of Trp53515C/515C MEFs by oncogenic Ras. (A) Transformation of Trp53515C/+p21−/− (C/+p21−/−), Trp53515C/515Cp21−/− (C/Cp21−/−), Trp53515C/515C (C/C), and Trp53−/− MEFs in cooperation with activated Ras was determined by a focus-forming assay. Passage-2 cells were infected with an Ha-RasV12 or control retroviral vector and plated with noninfected cells of the same genotype. Foci were counted after 15 days of culture. (B) Quantification of the number of foci arising in MEFs with different genotypes. Depicted are averages with SEs as determined from triplicate dishes from a representative experiment performed three times.
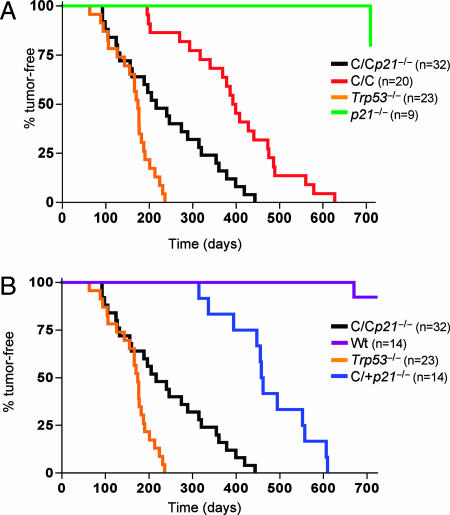
Because p53R172P is completely deficient for inducing apoptosis and loss of p21 completely abolished its cell cycle arrest function, Trp53515C/515Cp21−/− mice were monitored for survival and tumor incidence. Deletion of p21 significantly accelerated tumor incidence in Trp53515C/515C mice (Fig. 3A). The median survival of Trp53515C/515C mice was 395 days, whereas that of Trp53515C/515Cp21−/− mice was 233 days (P = 0.0001). Trp53515C/515Cp21−/− mice began to develop tumors at a rate similar to that of Trp53−/− mice, yet their overall survival was significantly prolonged (P = 0.001). Although 50% of Trp53515C/515Cp21−/− mice were tumor-bearing at 233 days, >80% of Trp53−/− mice had succumbed to tumorigenesis at this time point. The types of tumors arising in Trp53515C/515Cp21−/− mice resembled those of Trp53515C/515C and Trp53−/− mice being mainly composed of sarcomas and lymphomas (16, 21, 22). Consistent with previous data, 45% of Trp53515C/515C mice developed sarcomas, whereas 50% succumbed to lymphomas. In Trp53515C/515Cp21−/− mice, the incidence of sarcomas rose to 59%, and lymphomas were reduced to 31%. Trp53515C/515Cp21−/− animals developed different types of sarcomas, including angiosarcomas, spindle cell sarcomas, and anaphasic and biphasic sarcomas. Lymphomas were classified as diffuse and/or histiocytic. In addition, some Trp53515C/515Cp21−/− mice developed carcinomas (6%), and one had a benign adenoma (3%).
Fig. 3.
Loss of p21 accelerates tumor onset in Trp53515C/515C mice. Shown are tumor-free survival rates of Trp53515C/515C (C/C; n = 20), Trp53515C/515Cp21−/− (C/Cp21−/−; n = 32) Trp53−/− (n = 23), wild-type (Wt, n = 14), p21−/− (n = 9), and Trp53515C/+p21−/− (C/+p21−/−; n = 14) mice. Survival was calculated by using the Kaplan–Meier method.
The strain of p21−/− mice used in this study are not tumor-prone likely because of an intact apoptotic response (5). Accordingly, Trp53515C/+p21−/− mice, like p21−/− animals retaining a p53-dependent apoptotic response, had a prolonged survival rate compared with Trp53515C/515Cp21−/− mice that were deficient for apoptosis (Figs. 1D and 3B). These results indicate that p53-dependent apoptosis was essential for tumor suppression in the absence of p21 and revealed a cooperative relationship between p21 expression and p53-dependent apoptosis in suppression of tumorigenesis.
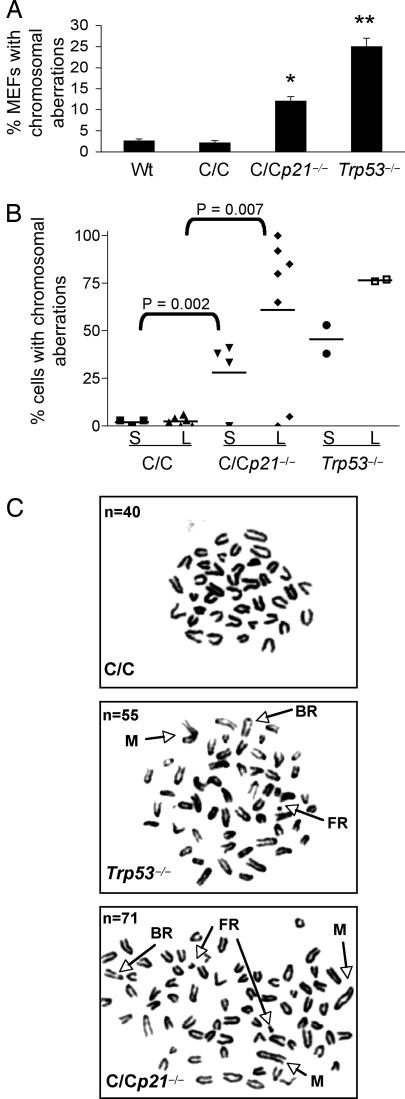
Because Trp53515C/515C MEFs and tumor cells contain diploid genomes (16), we also addressed the role of p21 in maintaining chromosomal stability. Metaphase spreads of early passage Trp53515C/515C, Trp53515C/515Cp21−/−, and Trp53−/− MEFs were surveyed for chromosomal aberrations in the absence of DNA damage. As previously reported (16), Trp53515C/515C MEFs, like wild-type cells, contained few chromosomal aberrations (2.6% and 3.0%, respectively) (Fig. 4A). However, 13% of Trp53515C/515Cp21−/− and 25% of Trp53−/− MEFs harbored chromosomal abnormalities. Thus, p21 coupled cell cycle control and maintenance of chromosomal stability.
Fig. 4.
Loss of p21 causes CIN in Trp53515C/515C MEFs and tumors. (A) Metaphase spreads from wild-type (Wt), Trp53515C/515C (C/C), Trp53515C/515Cp21−/− (C/Cp21−/−), and Trp53−/− MEFs at passage 2 were scored for chromosomal aberrations (n = 128 metaphases scored). ∗, P < 0.01; ∗∗, P < 0.005 (Student's t test). (B) Percentage of sarcoma (S) or lymphoma (L) tumor cells with chromosomal aberrations arising in p53 mutant mice. At least 30 cells per tumor sample were scored for chromosomal aberrations. P values were determined by Student's t test. (C) Representative metaphase spreads of lymphoma cells derived from p53 mutant mice. FR, fragment; BR, break; M, marker. n, total chromosome number.
To probe for CIN in tumors, cells from lymphomas and sarcomas arising in Trp53515C/515C and Trp53515C/515Cp21−/− mice were also analyzed for chromosomal aberrations. Metaphase spreads of Trp53515C/515C sarcomas (three cases) and lymphomas (four cases) revealed a diploid chromosome content and few chromosomal aberrations as previously published for lymphomas (16) (Fig. 4 B and C). However, three of four sarcomas and five of seven lymphomas from Trp53515C/515Cp21−/− mice had aneuploid genomes with overt chromosomal aberrations such as breaks, fusions, and marker chromosomes. These aberrations were similar to those in Trp53-null tumors. These results demonstrate that loss of p53-dependent apoptosis and p21 expression led to improper cell cycle control, development of CIN, and subsequent tumor development.
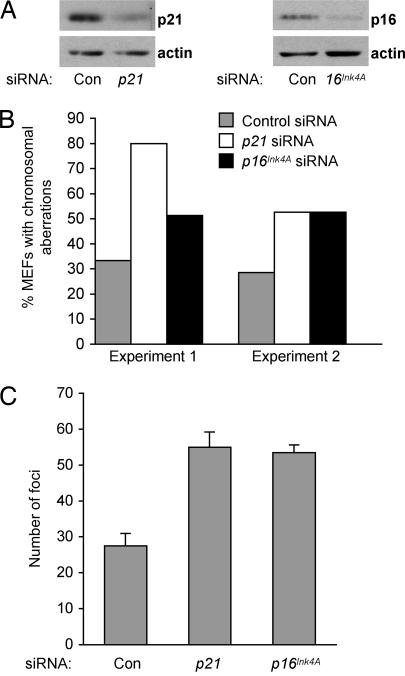
We have shown that p21 loss led to CIN and enhanced Ras-induced transformation of Trp53515C/515C MEFs. To determine whether these effects were specific to p21 or could be caused by disruption of other cell cycle regulators, we performed siRNA experiments to down-modulate p16Ink4A, which encodes another cyclin-dependent kinase inhibitor that regulates the G1/S transition (23, 24). Low passage Trp53515C/515C MEFs were transfected with control siRNA or siRNAs specific for p21 or p16Ink4A, and expression was confirmed by immunoblot analysis (Fig. 5A). To detect chromosomal alterations, cells were cultured for two additional passages after transfection with siRNAs and then prepared for chromosomal analysis. As with genetic ablation of p21, down-modulation of p21 by siRNA increased the incidence of chromosomal aberrations in Trp53515C/515C MEFs by 2- to 3-fold over cells transfected with control siRNA (Fig. 5B). Transfection with p16Ink4A siRNA also enhanced the abundance of cells with chromosomal aberrations approximately 2-fold over control cells. These results demonstrate that loss of p16Ink4A, like loss of p21, was sufficient to disrupt chromosomal stability in Trp53515C/515C MEFs.
Fig. 5.
p16Ink4A deficiency enhanced CIN and transformation of Trp53515C/515C cells. (A) Trp53515C/515C MEFs at passage 2 were transfected with the indicated siRNAs, and decreased protein levels were confirmed by immunoblot analysis. (B) Metaphase spreads of Trp53515C/515C MEFs transfected with the indicated siRNAs were scored for chromosomal aberrations. Depicted are the results obtained from two independent experiments in which duplicate dishes of MEFs were transfected with siRNAs then pooled for chromosomal analysis 6 days later. (C) Trp53515C/515C MEFs were infected with Ha-RasV12, transfected with the indicated siRNAs, and then plated 48 h later. Foci were counted at day 15. Con, control.
Because p16Ink4A is commonly inactivated in human tumors (25), we sought to determine whether p16Ink4A deficiency, like p21 loss, would bestow oncogenic potential to Trp53515C/515C MEFs. To this end, we performed focus-forming assays with Ha-RasV12-infected Trp53515C/515C MEFs transfected with p21, p16Ink4A, or control siRNAs. As in previous experiments, p21 loss caused a 2-fold increase in the number of Trp53515C/515C foci formed compared with control cells (Fig. 5C). Similarly, cells transfected with p16Ink4A siRNA also formed twice as many foci as did cells transfected with control siRNA. Thus, disruption of two critical cell cycle regulators permitted the development of CIN and increased transformation potential in the absence of p53-dependent apoptosis.
Although loss of p21 accelerated tumor onset in Trp53515C/515C mice, the survival of Trp53515C/515Cp21−/− and Trp53-null mice was significantly different (P = 0.001) (Fig. 3). We therefore surveyed gene expression by microarray analysis in an attempt to identify additional p53R172P-dependent genes that may have conferred a survival advantage to Trp53515C/515Cp21−/− mice. Wild-type, Trp53515C/515C, and Trp53-null MEFs were treated with γ-radiation and harvested 6 h after treatment. Gene expression profiles from irradiated wild-type and Trp53515C/515C MEFs were compared with profiles of irradiated Trp53-null MEFs. Twenty-nine and 18 genes were differentially expressed in wild-type and Trp53515C/515C MEFs, respectively, compared with Trp53-null MEFs according to the criteria detailed in Materials and Methods. Genes with a fold change ≥2 and previously characterized as p53 targets (26–30) or determined to have consensus p53 binding sites (31) are listed in Table 1. Three genes, p21, Ak1, and Wig1, were induced in both wild-type and Trp53515C/515C cells compared with Trp53-null cells. We confirmed p53R172P up-regulation of these genes by real-time RT-PCR analysis. Activation was determined by normalizing gene expression in irradiated wild-type, Trp53515C/515C, and Trp53515C/515Cp21−/− MEFs relative to irradiated Trp53-null MEFs. In response to γ-radiation, wild-type p53 caused an approximate 4-fold induction of Ak1 and Wig1 and a robust 80-fold induction of p21 over control cells (Fig. 6A). In Trp53515C/515C MEFs, p53R172P caused a 4-fold activation of Ak1 and Wig1 and a 10-fold activation of p21 (Fig. 6B). Trp53515C/515Cp21−/− MEFs exhibited a 4-fold induction of Ak1 and Wig1, but no change was detected in p21 expression (Fig. 6C). Activation of Ak1 and Wig1 by p53 can inhibit cellular proliferation (29, 32, 33). Our data suggest that these factors may also play important roles in p53 tumor suppression.
Table 1.
p53 target genes regulated by p53R172P
| Probe set | Description | FC/SE |
|---|---|---|
| Wild-type/Trp53−/− | ||
| 96801_at | Adenylate kinase 1 (Ak1) | 5.5/1.6 |
| 160127_at | Cyclin G | 8.8/1.7 |
| 98067_at | Cyclin-dependent kinase inhibitor 1A (p21) | 5.0/2.2 |
| 101587_at | Epoxide hydrolase 1, microsomal | 13.1/0 |
| 99629_at | Etoposide-induced 2.4 mRNA | 3.1/1.1 |
| 100064_at | Gap junction membrane channel protein α 1 | 2.5/0.1 |
| 98501_at | IL-1 receptor-like 1 | 2.6/0 |
| 99622_at | Kruppel-like factor 4 (gut) | 2.2/0.4 |
| 99638_at | Procollagen, type XV | 3.9/1.0 |
| 98110_at | Transformed mouse 3T3 cell double minute 2 | 3.5/0 |
| 92262_at | Wild-type p53-induced gene 1 (Wig1) | 4.3/0.9 |
| Trp53C/C/Trp53−/− | ||
| 96801_at | Adenylate kinase 1 (Ak1) | 3.3/1.1 |
| 93536_at | Bcl-2 associated X protein | 2.4/0.3 |
| 95423_at | Calcium-binding protein P22 | 2.2/0.3 |
| 98067_at | Cyclin-dependent kinase inhibitor 1A (p21) | 4.8/2.1 |
| 94881_at | Cyclin-dependent kinase inhibitor 1A (p21) | 9.4/3.6 |
| 92262_at | Wild-type p53-induced gene 1 (Wig1) | 3.7/1.3 |
FC/SE, fold change/standard error.
Fig. 6.
Real-time RT-PCR analysis for transcriptional activation by p53R172P. Gene expression was first normalized to Gapdh. Fold induction was calculated as gene expression differences in irradiated (6 Gy) wild-type (A), Trp53515C/515C (B), and Trp53515C/515Cp21−/− (C) MEFs over that in irradiated Trp53-null cells. Data are depicted as the fold induction with SE from triplicate samples.
Discussion
By exploiting the properties of a p53 point mutant, p53R172P, we have illustrated the importance of separate p53 activities in delaying tumor onset. Our analyses show that cooperation between p21 and apoptosis was essential for tumor suppression by p53. Mechanistically, p21 was required for cell cycle arrest and preservation of chromosomal stability. Trp53515C/515Cp21−/− MEFs, like Trp53−/− cells, had a defective G1 checkpoint and developed chromosomal aberrations. Loss of p21 also enhanced transformation of Trp53515C/515C MEFs in response to oncogenic Ras, whereas Trp53515C/+p21−/− cells, retaining a single wild-type p53 allele, resisted Ras-induced transformation. These data demonstrate that a defective p53 cell cycle arrest due to the absence of p21 imparted a proliferative advantage to cells, contributing to CIN. However, CIN was resolved in cells with an intact p53-depedendent apoptotic response. Additionally, a functional p53-dependent apoptosis pathway in p21−/− mice was associated with their tumor-free survival, whereas Trp53515C/515Cp21−/− and Trp53−/− mice succumbed to early onset tumorigenesis and tumors arising in these mice were characterized by CIN. Thus, p53-dependent apoptosis played an obligate role in preventing expansion of aneuploid cell populations and tumor development.
That Trp53515C/515C MEFs formed fewer foci than Trp53−/− cells in response to oncogenic Ras raised the possibility that p53R172P can induce replicative senescence. These data would implicate replicative senescence in tumor suppression by p53R172P.
Various studies have implicated p53 in the protection against tumor development in the context of CIN (3, 9, 34, 35). Deletion of checkpoint or DNA repair genes leads to CIN but not tumorigenesis unless p53 also is removed. Our analyses of a different cyclin-dependent kinase inhibitor suggest that loss of p53-dependent apoptosis also would enhance the tumor predisposition of p16Ink4A-null mice. This result would implicate separate p53 functions in distinct tumor suppressor pathways.
By use of an apoptosis-deficient mouse model, our findings establish that tumor development resulting from CIN relies, specifically, upon abrogation of the p53 apoptotic pathway. In summary, we have shown that p53 cell cycle control through p21 is critical for suppressing tumorigenesis by preservation of chromosomal stability. Although p53 may serve as a gatekeeper through its apoptotic function (36), its activation of p21 fulfills its role as guardian of the genome (37).
Materials and Methods
Mice and Tumor Analysis.
Generation of Trp53515C/515C mice was previously described (16). p21−/− mice were obtained from T. Jacks (Massachusetts Institute of Technology, Cambridge, MA) and were crossed with C57BL/6 mice for more than five generations until the background was at >90% C57BL/6. The background of wild-type and Trp53−/− mice was >90% C57BL/6. To detect chromosomal aberrations, lymphoma cells were isolated from affected spleens or lymph nodes by homogenization. Sarcoma cells were prepared by homogenizing tissues with trypsin for 5 min at 37°C then incubating them with 4 mg/ml collagenase D and dispase (Sigma, St. Louis, MO) for 2 h and plated in complete media. Metaphase spreads were prepared and evaluated as described (38).
Cell Culture and Apoptosis Assay.
MEFs were generated from 13.5 day-old embryos. For cell cycle analysis, MEFs were treated as previously described (16). Focus-forming assays were performed as described (39) with minor modifications. After selection of MEFs with puromycin, 2,000 puromycin-resistance cells were mixed with 300,000 noninfected cells of the same genotype and plated in 100-mm dishes. Fifteen days after plating, cells were fixed and stained with crystal violet in methanol, and foci were counted. For growth curve analysis, 19,000 cells were plated in triplicate in 35-mm dishes and counted at the indicated time points. The medium was changed every 3 days. Cells were transfected with siRNAs for p21 (40), p16Ink4a (40) or with control siRNA (Dharmacon, Lafayette, CO) by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. For apoptosis assays, freshly isolated thymocytes were prepared and evaluated as previously described (16).
Immunoblot Analysis.
Cell pellets were resuspended in lysis buffer (41). Forty micrograms of protein were resolved on 10% polyacrylamide gels and transferred to nitrocellulose. Membranes were incubated with anti-p53 (CM5; Novocastra Laboratories, Newcastle upon Tyne, U.K.), monoclonal anti-p21 (BD Pharmingen, San Diego, CA), anti-p16 (M-156; Santa Cruz Biotechnologies, Santa Cruz, CA), or anti-β-actin antibodies (Sigma).
Real-Time RT-PCR.
MEFs were exposed to 6 Gy of γ-radiation with a 137Cs source then cultured for 6 h and prepared as described (42). The primer sequences for p21 and Gapdh were previously described (42). The following primer sets were also used: Ak1, GGAGACCATCAAGAAGCGGC and TTCGGCATTGACCTTGCG; and Wig1, CTACTGTAAGCTGTGCGATGCC and AGTGACTCTGAGCTTCGGCC. Expression of mRNA was normalized to expression of Gapdh in each reaction.
Microarray Analysis.
MEFs were treated with 6 Gy of γ-radiation and harvested 6 h later. Total RNA was isolated by using an RNeasy kit (Qiagen, Valencia, CA). Ten micrograms of total RNA was used for cRNA probe preparation and hybridization onto oligonucleotide U74Av2 GeneChip arrays according to manufacturer's recommendations (Affymetrix, Santa Clara, CA). Hybridized arrays were scanned with a GeneArray Scanner (Hewlett–Packard, Palo Alto, CA). The image and intensity data were collected with Affymetrix Microarray Suite 5.0 and further analyzed by DNA-Chip Analyzer software (dChip) (43). Briefly, the scanned images were quantified with Microarray Suite 5.0 and then linearly scaled to an average expression level of 2,500 units to generate a signal intensity for each probe set. The signal intensities from the 11 or 16 probe pairs for each gene were used to determine gene expression values and differentially expressed genes with dChip. The data were first normalized against a default baseline array and expression values were calculated by using the perfect-match– mismatch model. Gene expression was considered significantly altered if all of the following conditions were met: (i) expression value must be called present in at least one of the paired samples for comparison; (ii) the difference of expression values between the paired samples was ≥100 to avoid the effects of unreliable low intensity; (iii) the ratio of expression values between the paired samples exceeds a threshold more than or equal to 1.5 or less than or equal to −1.5, with a lower confidence bound of the 90% confidence interval [lower bound of fold change (LBFC)]; (iv) the paired samples were significantly different, with P values ≤0.05 as determined by t test. The irradiation and microarray hybridization were repeated in two independent experiments, and the two independent data sets were analyzed by two strict strategies. First, the two data sets were combined to identify differentially expressed genes by using the above comparison criteria. Second, the two data sets were separately analyzed by using the same comparison criteria, and the differentially expressed genes in common between the two independent analyses were identified. The first strategy generated a single LBFC for each gene. The second strategy generated two LBFCs for each overlapping gene. The two LBFCs generated by the second strategy were averaged and a SE was obtained.
Acknowledgments
We thank Tomoo Iwakuma, Tamara Terzian, and Yasmine Valentin-Vega for guidance and helpful discussions and Sean Post for careful review of the manuscript. This study was supported by National Institutes of Health Grant CA82577 (to G. Lozano). Veterinary support, cytogenetics, and Affymetrix core facilities were supported by National Cancer Institute Cancer Center Support Grant CA16672. J.A.B. was supported by National Institutes of Health Cancer Genetics Training Grant CA009299.
Abbreviations
- MEF
mouse embryo fibroblast
- CIN
chromosomal instability.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The microarray data reported in this paper have been deposited in the ArrayExpress database (accession no. E-MEXP-917).
References
- 1.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 2.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 3.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 4.Waldman T, Kinzler KW, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 5.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 6.Brugarolas J, Bronson RT, Jacks T. J Cell Biol. 1998;141:503–514. doi: 10.1083/jcb.141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y. Mol Cell Biol. 2000;20:6147–6158. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebel M, Cardiff RD, Leder P. Cancer Res. 2001;61:1816–1819. [PubMed] [Google Scholar]
- 9.Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, et al. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 10.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, et al. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 11.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 12.Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig RL, Bates S, Vousden KH. Mol Cell Biol. 1996;16:4952–4960. doi: 10.1128/mcb.16.9.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowan S, Ludwig RL, Haupt Y, Bates S, Lu X, Oren M, Vousden KH. EMBO J. 1996;15:827–838. [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G. Nat Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 17.Hundley JE, Koester SK, Troyer DA, Hilsenbeck SG, Subler MA, Windle JJ. Mol Cell Biol. 1997;17:723–731. doi: 10.1128/mcb.17.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozano G, Zambetti GP. J Pathol. 2005;205:206–220. doi: 10.1002/path.1704. [DOI] [PubMed] [Google Scholar]
- 19.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier MS, Aizawa S, Mak TW, Taniguchi T. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 21.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 22.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 23.Serrano M, Hannon GJ, Beach D. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 24.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 25.Ruas M, Peters G. Biochim Biophys Acta. 1998;1378:F115–F177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 26.Miled C, Pontoglio M, Garbay S, Yaniv M, Weitzman JB. Cancer Res. 2005;65:5096–5104. doi: 10.1158/0008-5472.CAN-04-4232. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varmeh-Ziaie S, Okan I, Wang Y, Magnusson KP, Warthoe P, Strauss M, Wiman KG. Oncogene. 1997;15:2699–2704. doi: 10.1038/sj.onc.1201454. [DOI] [PubMed] [Google Scholar]
- 29.Collavin L, Lazarevic D, Utrera R, Marzinotto S, Monte M, Schneider C. Oncogene. 1999;18:5879–5888. doi: 10.1038/sj.onc.1202970. [DOI] [PubMed] [Google Scholar]
- 30.Madden SL, Galella EA, Riley D, Bertelsen AH, Beaudry GA. Cancer Res. 1996;56:5384–5390. [PubMed] [Google Scholar]
- 31.Hoh J, Jin S, Parrado T, Edington J, Levine AJ, Ott J. Proc Natl Acad Sci USA. 2002;99:8467–8472. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellborg F, Qian W, Mendez-Vidal C, Asker C, Kost-Alimova M, Wilhelm M, Imreh S, Wiman KG. Oncogene. 2001;20:5466–5474. doi: 10.1038/sj.onc.1204722. [DOI] [PubMed] [Google Scholar]
- 33.Israeli D, Tessler E, Haupt Y, Elkeles A, Wilder S, Amson R, Telerman A, Oren M. EMBO J. 1997;16:4384–4392. doi: 10.1093/emboj/16.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, Harris CC, Deng CX. Nat Genet. 2001;28:266–271. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
- 36.Levine AJ. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 37.Lane DP. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 38.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, Kiyokawa H. Genes Dev. 2002;16:2923–2934. doi: 10.1101/gad.1033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapoor M, Lozano G. Proc Natl Acad Sci USA. 1998;95:2834–2837. doi: 10.1073/pnas.95.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwakuma T, Parant JM, Fasulo M, Zwart E, Jacks T, de Vries A, Lozano G. Oncogene. 2004;23:7644–7650. doi: 10.1038/sj.onc.1207793. [DOI] [PubMed] [Google Scholar]
- 43.Li C, Wong WH. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]