Abstract
1. A search was made for an assay tissue with selective sensitivity to vasopressin. Of those smooth muscle preparations tested, the longitudinal muscle of the isolated rectum of the rabbit was the most satisfactory.
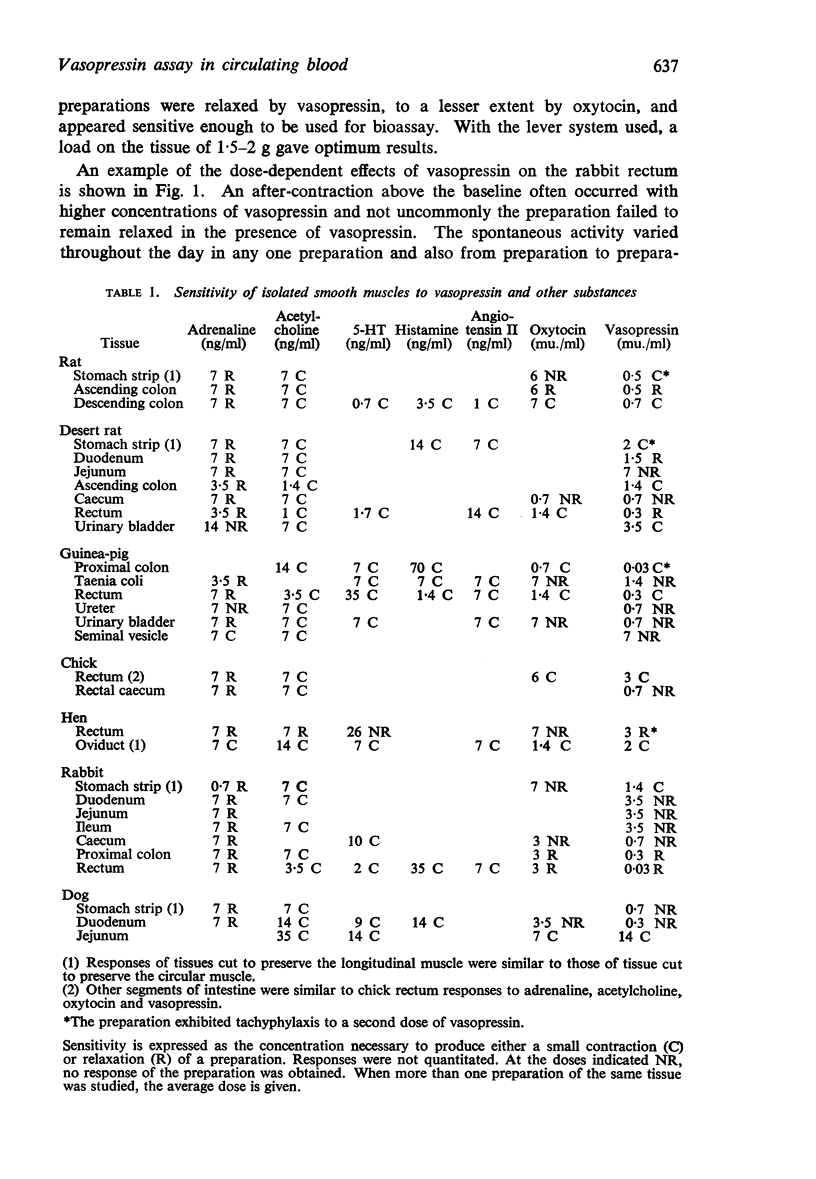
2. The rabbit isolated rectum, bathed in Krebs solution, was contracted by acetylcholine, angiotensin II amide, bradykinin and 5-hydroxytryptamine. It was relaxed by vasopressin, oxytocin and the catecholamines.
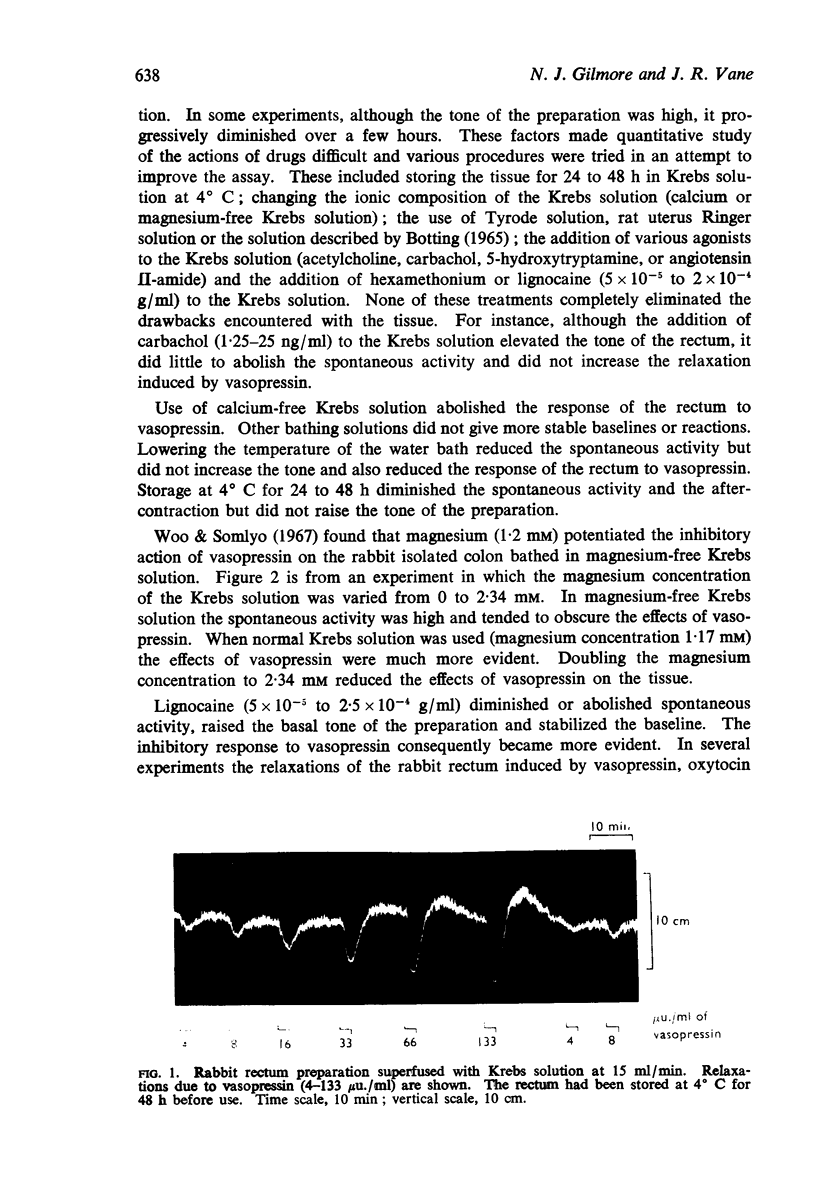
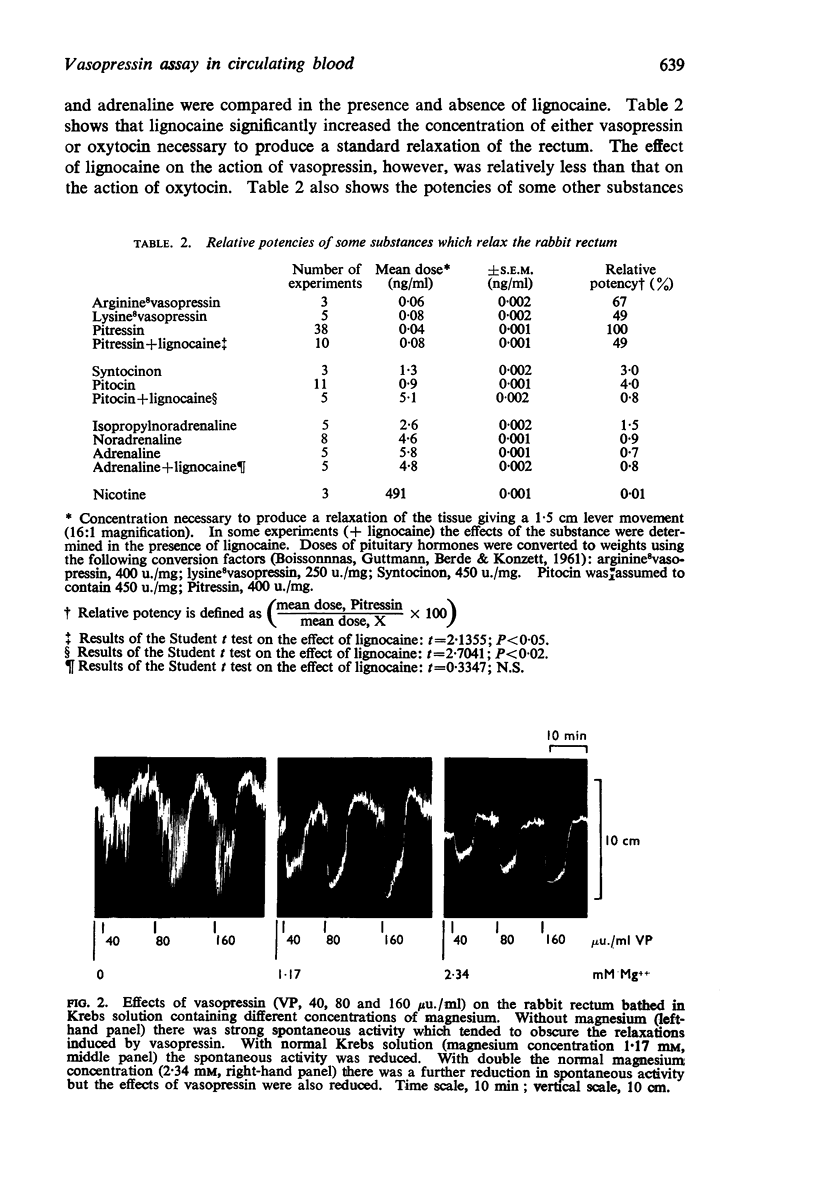
3. Vasopressin was active in concentrations of 4-100 μu./ml (0·01-0·25 ng/ml) and was 20-30 times more active than oxytocin. Bretylium had no effect on the relaxant action of vasopressin; nor did concentrations of α- and β-adrenoceptor blocking agents sufficient to abolish the actions of catecholamines. Lignocaine reduced the sensitivity of the rabbit rectum to both vasopressin and oxytocin without altering the actions of adrenaline. High concentrations of either vasopressin or oxytocin desensitized the rabbit rectum to the actions of both hormones, without affecting the actions of adrenaline. It was concluded that vasopressin and oxytocin act on a common population of receptors different from those for catecholamines.
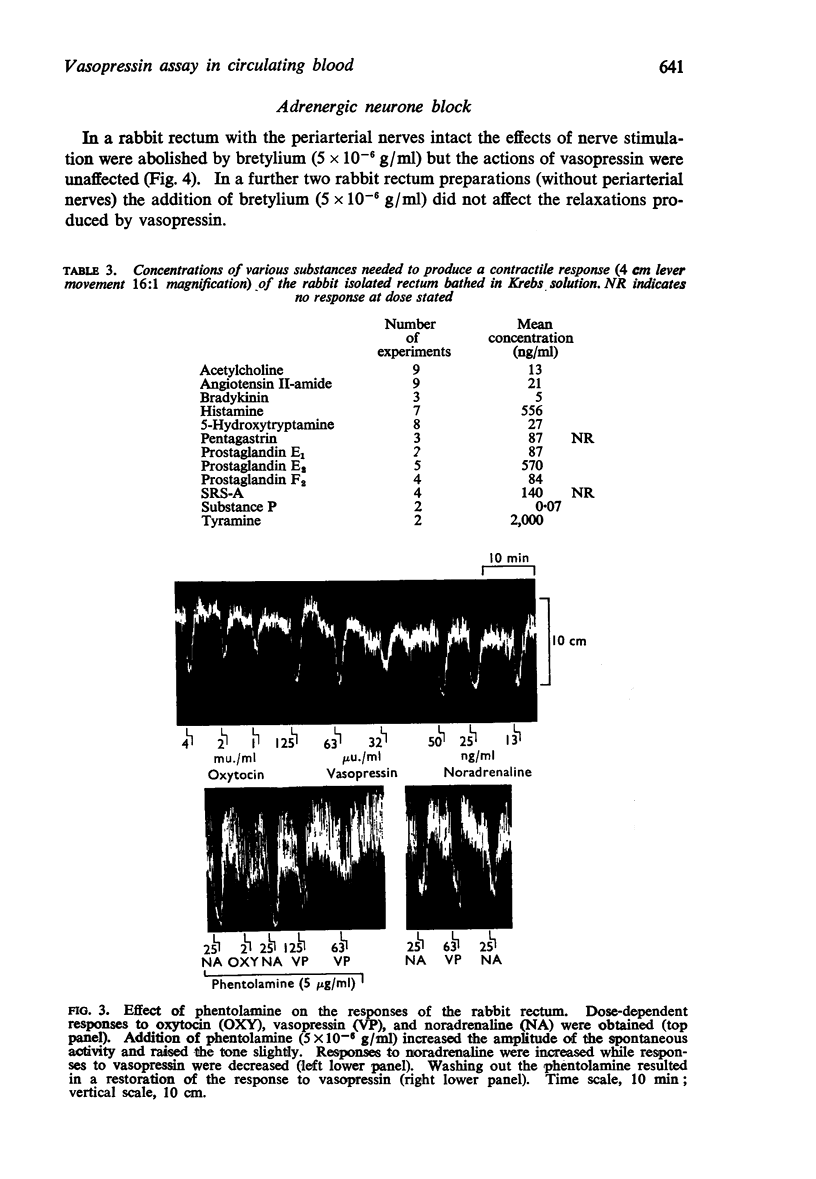
4. Phentolamine, unlike other α-adrenoceptor antagonists, reduced the relaxant action of vasopressin on the rectum.
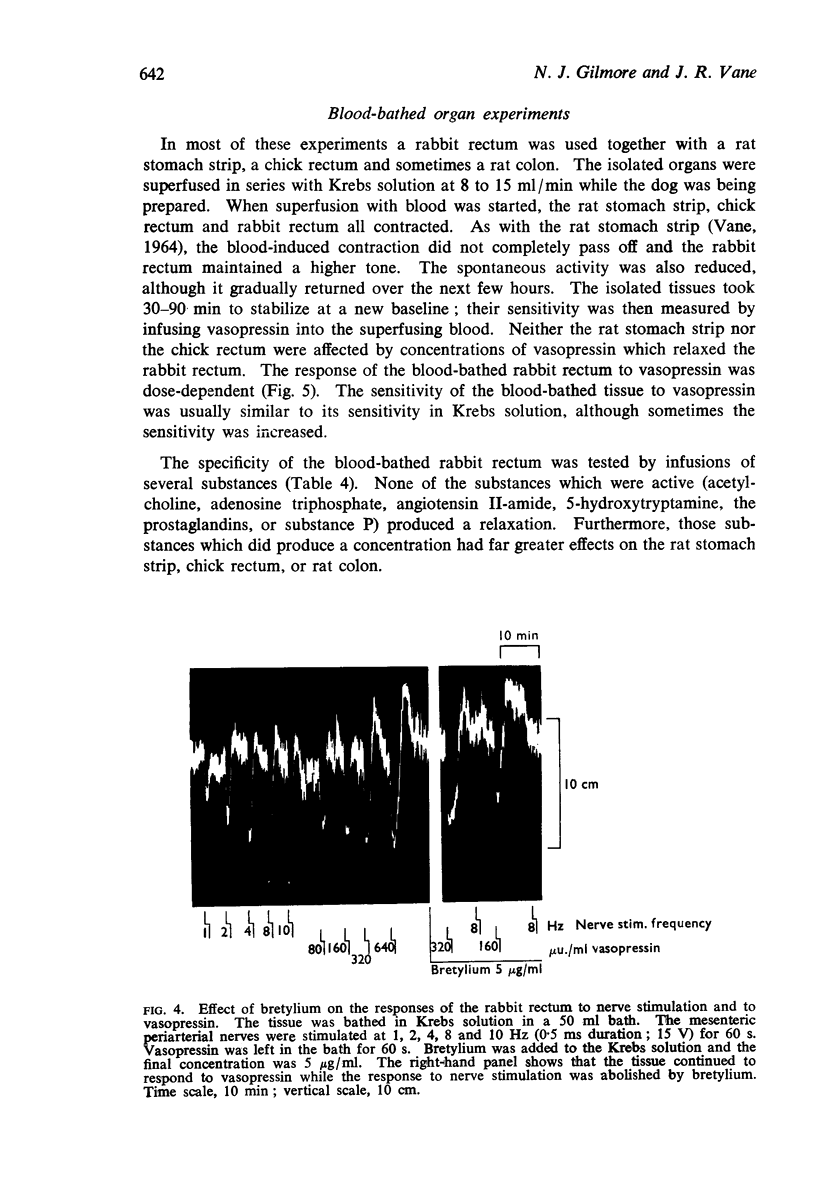
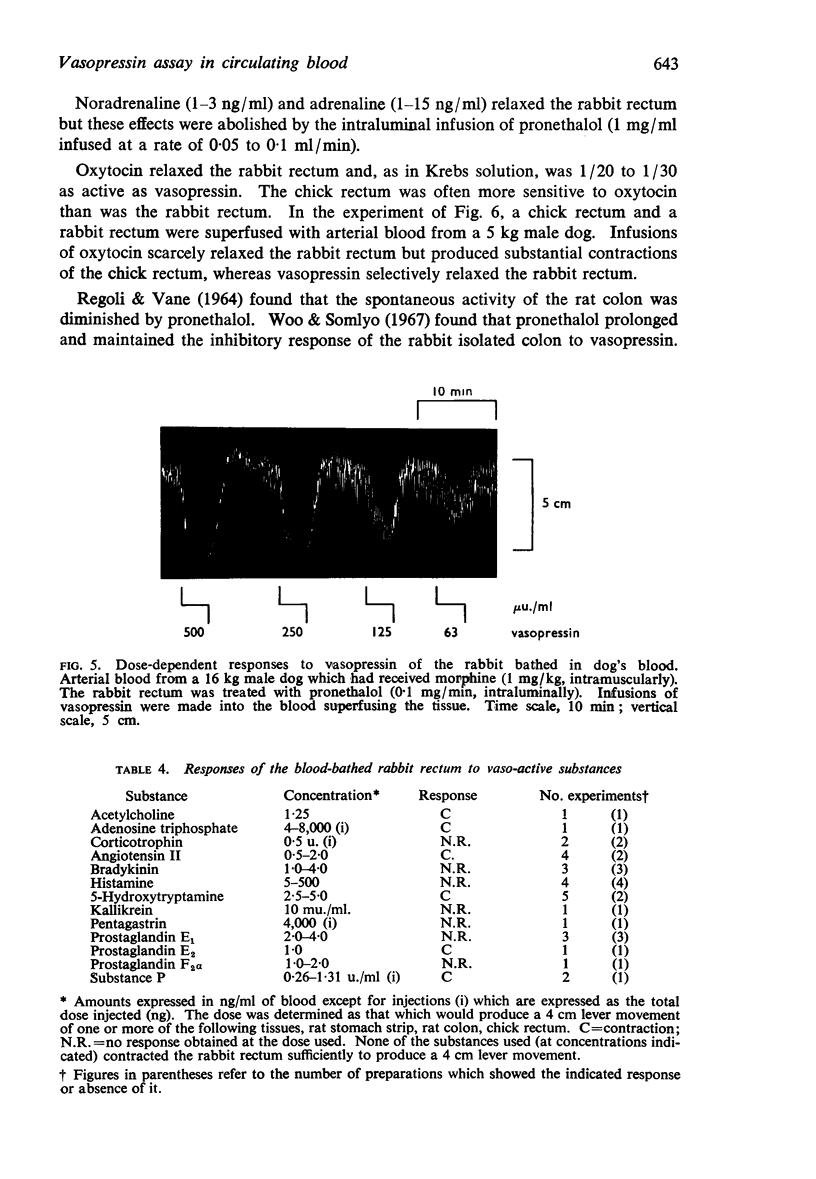
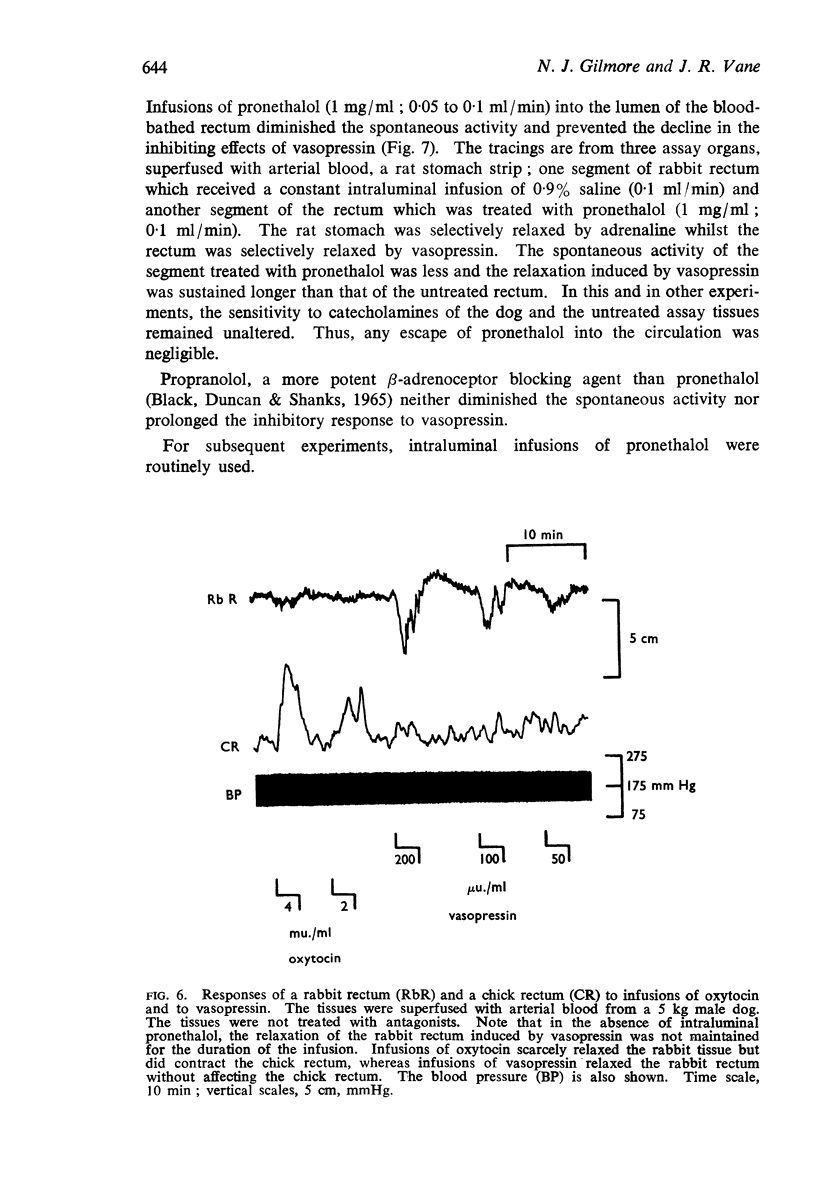
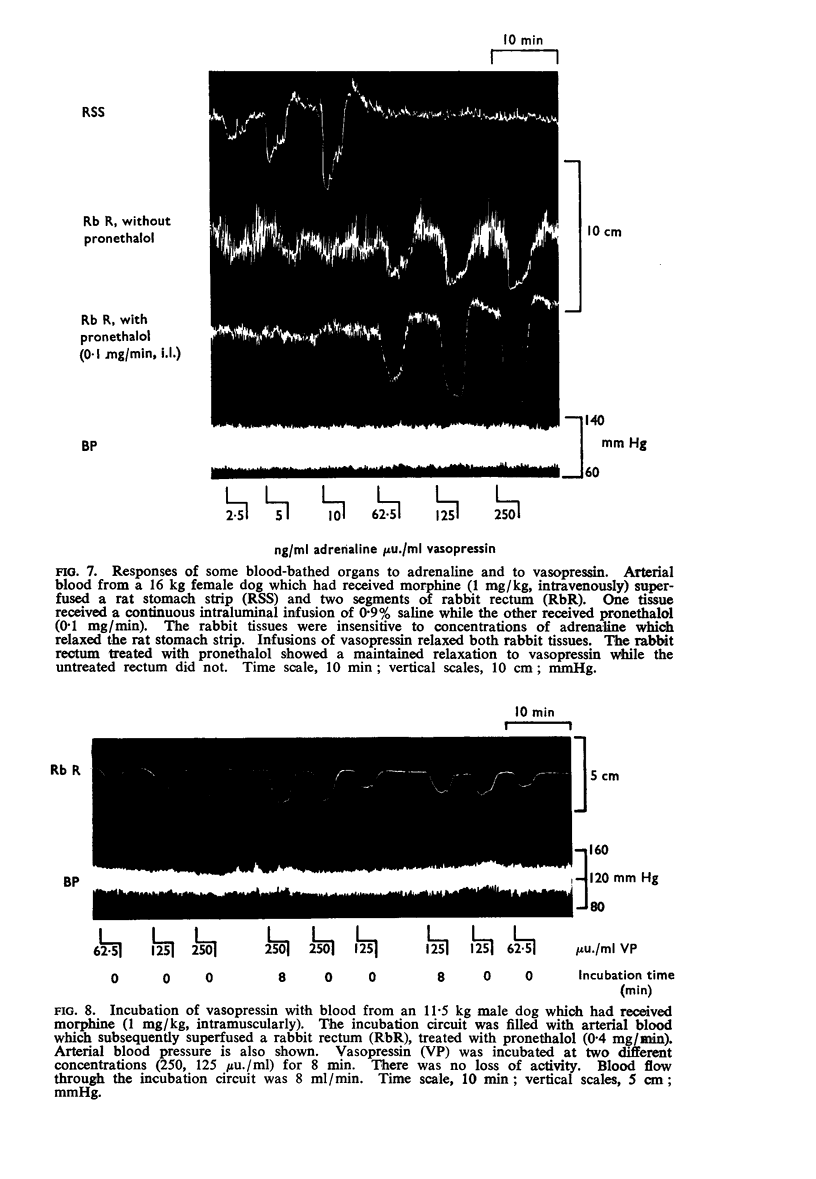
5. When superfused with blood from an anaesthetized dog, the rabbit rectum maintained a higher tone than in Krebs solution; it retained its sensitivity to vasopressin. Pronethalol, administered intraluminally, reduced spontaneous movement and abolished the actions of low concentrations of catecholamines, thereby increasing the specificity of the assay. No other substance tested relaxed the rectum in concentrations likely to be found in blood.
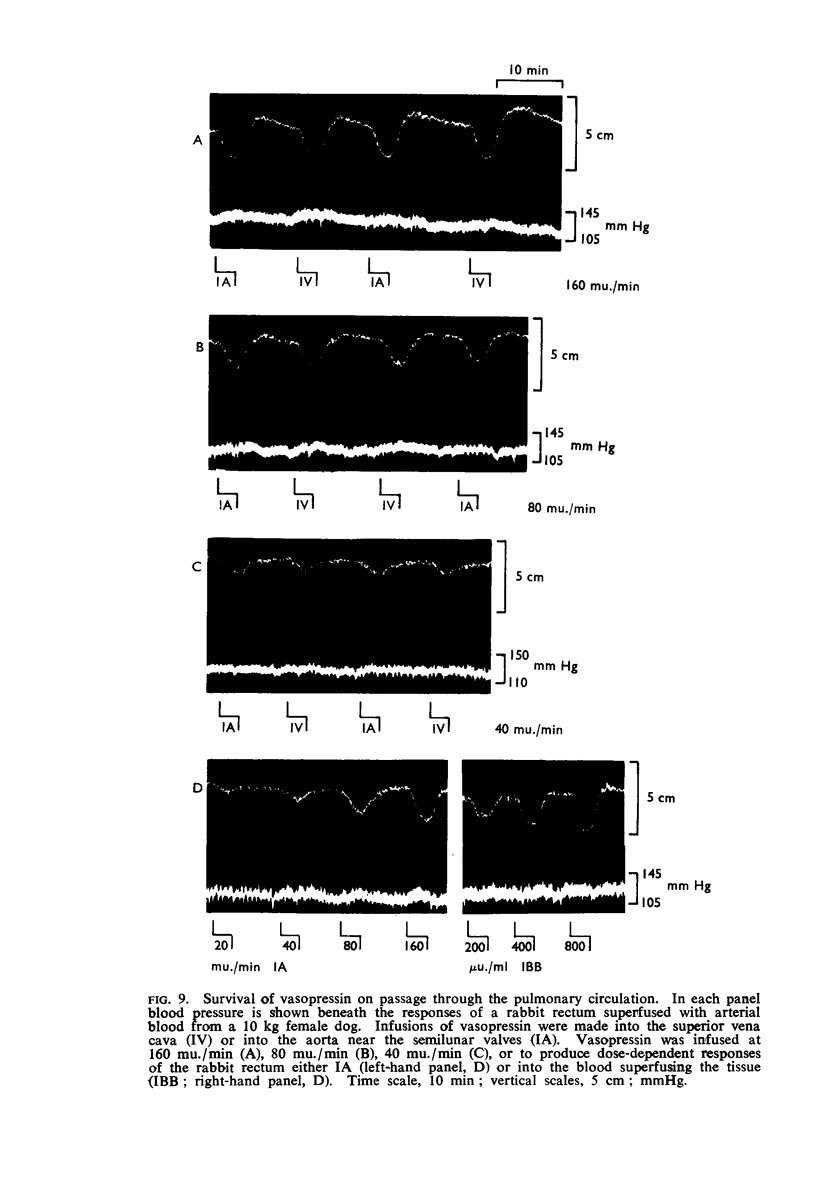
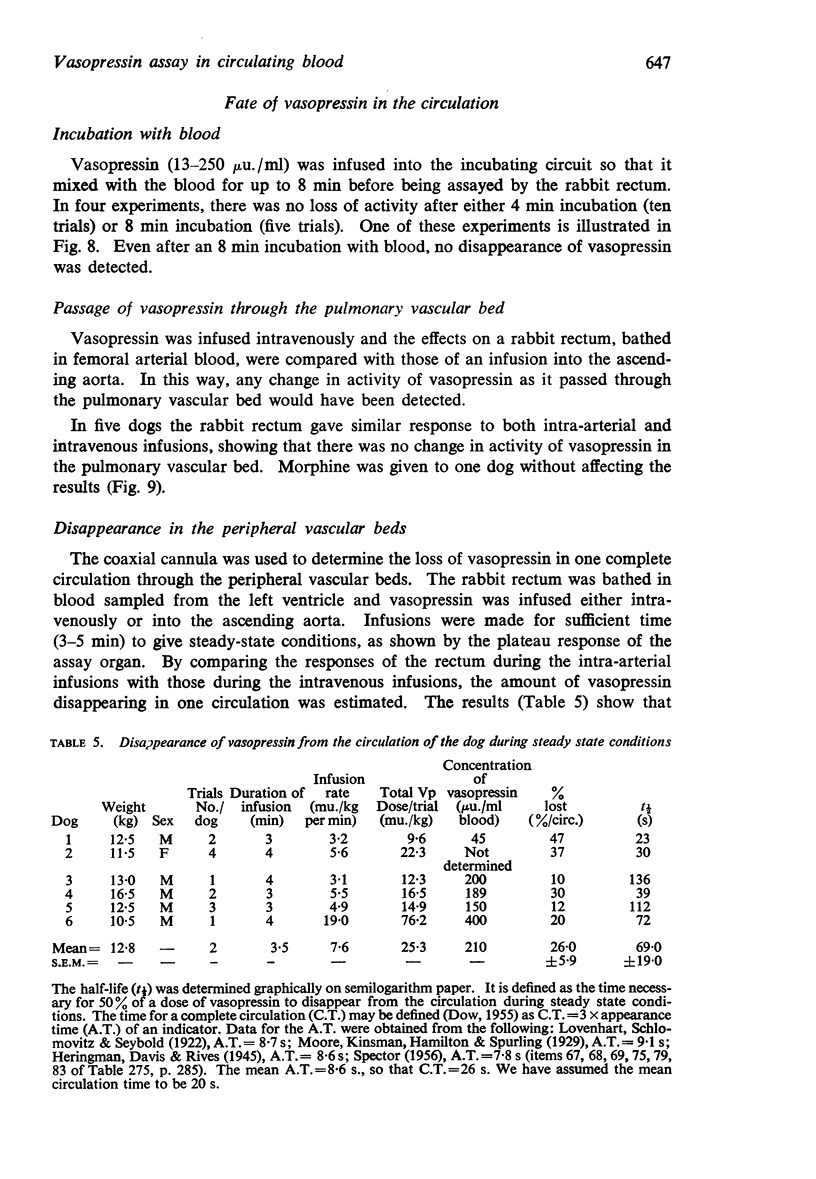
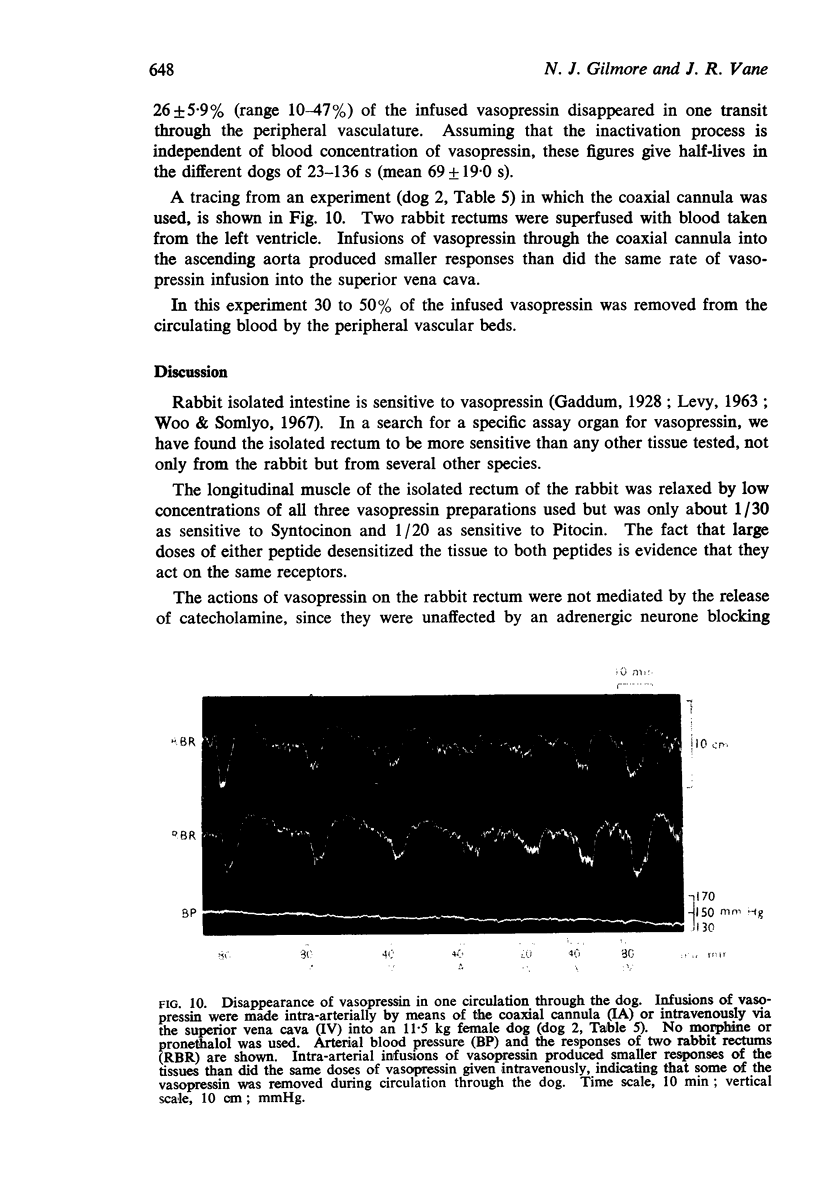
6. Vasopressin was stable in dog's blood; it survived passage through the pulmonary vascular bed; it had a half-life in the circulation of about 1 min.
7. The half-life of vasopressin in the circulation may depend upon the duration of the infusion.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N., KAVANAGH L., WHITING J. EFFECT OF MECHANICAL STIMULATION ON RABBITS' EYES: RELEASE OF ACTIVE SUBSTANCE IN ANTERIOR CHAMBER PERFUSATES. J Physiol. 1965 Feb;176:378–408. doi: 10.1113/jphysiol.1965.sp007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTTING J. H. AN ISOLATED PREPARATION WITH A SELECTIVE SENSITIVITY TO VASOPRESSIN. Br J Pharmacol Chemother. 1965 Feb;24:156–162. doi: 10.1111/j.1476-5381.1965.tb02090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Lewis G. P. The mechanism of the inhibitory action of adrenaline on the mammary gland. Br J Pharmacol Chemother. 1967 Nov;31(3):550–559. doi: 10.1111/j.1476-5381.1967.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Shanks R. G. Comparison of some properties of pronethalol and propranolol. Br J Pharmacol Chemother. 1965 Dec;25(3):577–591. doi: 10.1111/j.1476-5381.1965.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEUGH J., GADDUM J. H., HOLTON P., LEACH E. Assay of substance P on the fowl rectal caecum. Br J Pharmacol Chemother. 1961 Aug;17:144–158. doi: 10.1111/j.1476-5381.1961.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZACZKES J. W., KLEEMAN C. R. THE EFFECT OF VARIOUS STATES OF HYDRATION AND THE PLASMA CONCENTRATION ON THE TURNOVER OF ANTIDIURETIC HORMONE IN MAMMALS. J Clin Invest. 1964 Aug;43:1649–1658. doi: 10.1172/JCI105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E. A method for the assay of very small amounts of antidiuretic activity with a note on the antidiuretic titre of rat's blood. J Physiol. 1953 Oct;122(1):149–157. doi: 10.1113/jphysiol.1953.sp004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOW P. Dimensional relationships in dye-dilution curves from humans and dogs, with an empirical formula for certain troublesome curves. J Appl Physiol. 1955 Jan;7(4):399–408. doi: 10.1152/jappl.1955.7.4.399. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Half-lives of peptides and amines in the circulation. Nature. 1967 Sep 16;215(5107):1237–1240. doi: 10.1038/2151237a0. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. The detection and estimation of bradykinin in the circulating blood. Br J Pharmacol Chemother. 1967 Mar;29(3):367–377. doi: 10.1111/j.1476-5381.1967.tb01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADDUM J. H. Measurement of epinephrine, norepinephrine and related compounds; bioassay procedures. Pharmacol Rev. 1959 Jun;11(2 Pt 2):241–251. [PubMed] [Google Scholar]
- GADDUM J. H. The technique of superfusion. Br J Pharmacol Chemother. 1953 Sep;8(3):321–326. doi: 10.1111/j.1476-5381.1953.tb00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRY R. C., GILLESPIE J. S. An in vitro preparation of the distal colon of the rabbit with orthosympathetic and parasympathetic innervation. J Physiol. 1954 Mar 29;123(3):60P–61P. [PMC free article] [PubMed] [Google Scholar]
- Gaddum J. H. Some properties of the separated active principles of the pituitary (posterior lobe). J Physiol. 1928 Aug 14;65(4):434–440. doi: 10.1113/jphysiol.1928.sp002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey N., Jones J. J., Lee J. The renal clearance and plasma binding of vasopressin in the dog. J Endocrinol. 1967 Jun;38(2):163–171. doi: 10.1677/joe.0.0380163. [DOI] [PubMed] [Google Scholar]
- Heller H., Urban F. F. The fate of the antidiuretic principle of postpituitary extracts in vivo and in vitro. J Physiol. 1935 Dec 16;85(4):502–518. doi: 10.1113/jphysiol.1935.sp003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge R. L., Lowe R. D., Vane J. R. The effects of alteration of blood-volume on the concentration of circulating angiotensin in anaesthetized dogs. J Physiol. 1966 Aug;185(3):613–626. doi: 10.1113/jphysiol.1966.sp008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUSON H. D., BOCANEGRA M., BEUZEVILLE C. F. HEPATIC AND RENAL CLEARANCE OF VASOPRESSIN FROM PLASMA OF DOGS. Am J Physiol. 1965 Jul;209:199–214. doi: 10.1152/ajplegacy.1965.209.1.199. [DOI] [PubMed] [Google Scholar]
- LAUSON H. D., BOCANEGRA M. Clearance of exogenous vasopressin from plasma of dogs. Am J Physiol. 1961 Mar;200:493–497. doi: 10.1152/ajplegacy.1961.200.3.493. [DOI] [PubMed] [Google Scholar]
- LEVY B. The intestinal inhibitory response to oxytocin, vasopressin and bradykinin. J Pharmacol Exp Ther. 1963 Jun;140:356–366. [PubMed] [Google Scholar]
- Lawson W. R., Mackenna B. R. The inhibitory adrenergic receptors of the rabbit intestine. J Physiol. 1969 May;202(1):35P–36P. [PubMed] [Google Scholar]
- MANN M., WEST G. B. The nature of hepatic and splenic sympathin. Br J Pharmacol Chemother. 1950 Jun;5(2):173–177. doi: 10.1111/j.1476-5381.1950.tb01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikos E., Vane J. R. Effects of gastrin and its analogues on isolated smooth muscles. Nature. 1967 Apr 1;214(5083):105–107. doi: 10.1038/214105a0. [DOI] [PubMed] [Google Scholar]
- PATON W. D., VANE J. R. Analysis of he responses of the isolated stomach to electrical stimulation and to drugs. J Physiol. 1963 Jan;165:10–46. doi: 10.1113/jphysiol.1963.sp007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., VANE J. R. A SENSITIVE METHOD FOR THE ASSAY OF ANGIOTENSIN. Br J Pharmacol Chemother. 1964 Oct;23:351–359. doi: 10.1111/j.1476-5381.1964.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E Silva M., Jr, Rosenberg M. The release of vasopressin in response to haemorrhage and its role in the mechanism of blood pressure regulation. J Physiol. 1969 Jun;202(3):535–557. doi: 10.1113/jphysiol.1969.sp008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARE L. Rate of disappearance of arginine vasopressin from circulating blood in the dog. Am J Physiol. 1962 Dec;203:1179–1181. doi: 10.1152/ajplegacy.1962.203.6.1179. [DOI] [PubMed] [Google Scholar]
- SILVER L., SCHWARTZ I. L., FONG C. T., DEBONS A. F., DAHL L. K. Disappearance of plasma radioactivity after injection of H3- or I-131-labeled arginine vasopressin. J Appl Physiol. 1961 Nov;16:1097–1099. doi: 10.1152/jappl.1961.16.6.1097. [DOI] [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANE J. R. THE USE OF ISOLATED ORGANS FOR DETECTING ACTIVE SUBSTANCES IN THE CIRCULATING BLOOD. Br J Pharmacol Chemother. 1964 Oct;23:360–373. doi: 10.1111/j.1476-5381.1964.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C. Y., Somlyo A. P. Interaction of magnesium with vasopressin intestinal smooth muscle. J Pharmacol Exp Ther. 1967 Feb;155(2):357–366. [PubMed] [Google Scholar]


