Abstract
1. The prostaglandins are C20 unsaturated fatty acids which exhibit diverse physiological effects of short duration. We have investigated the speed of removal of PGE1 and PGF1α from the circulating blood and their subsequent metabolism by the isolated perfused rat liver.
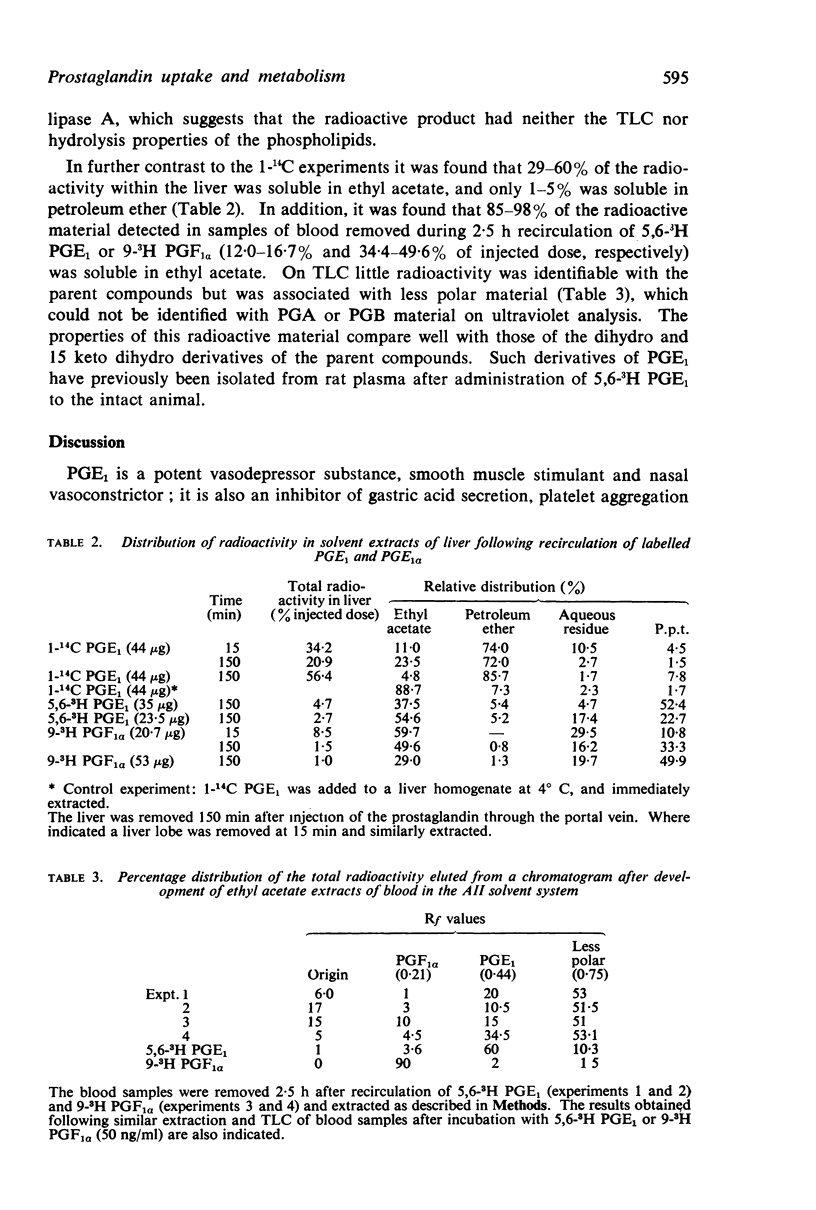
2. Following either a single injection of radiolabelled PGE1 or PGF1α into the hepatic artery or portal vein, or recirculation of prostaglandins through the liver for 2·5 h, the distribution of radioactivity within extracts of bile, blood and liver was determined. The nature of the radioactive products of meta-bolism was inferred by comparison of the distribution of radioactivity after injecting carbon and tritium labelled standards, and by thin-layer chromatography, gas-liquid chromatography, ultraviolet and bioassay analysis.
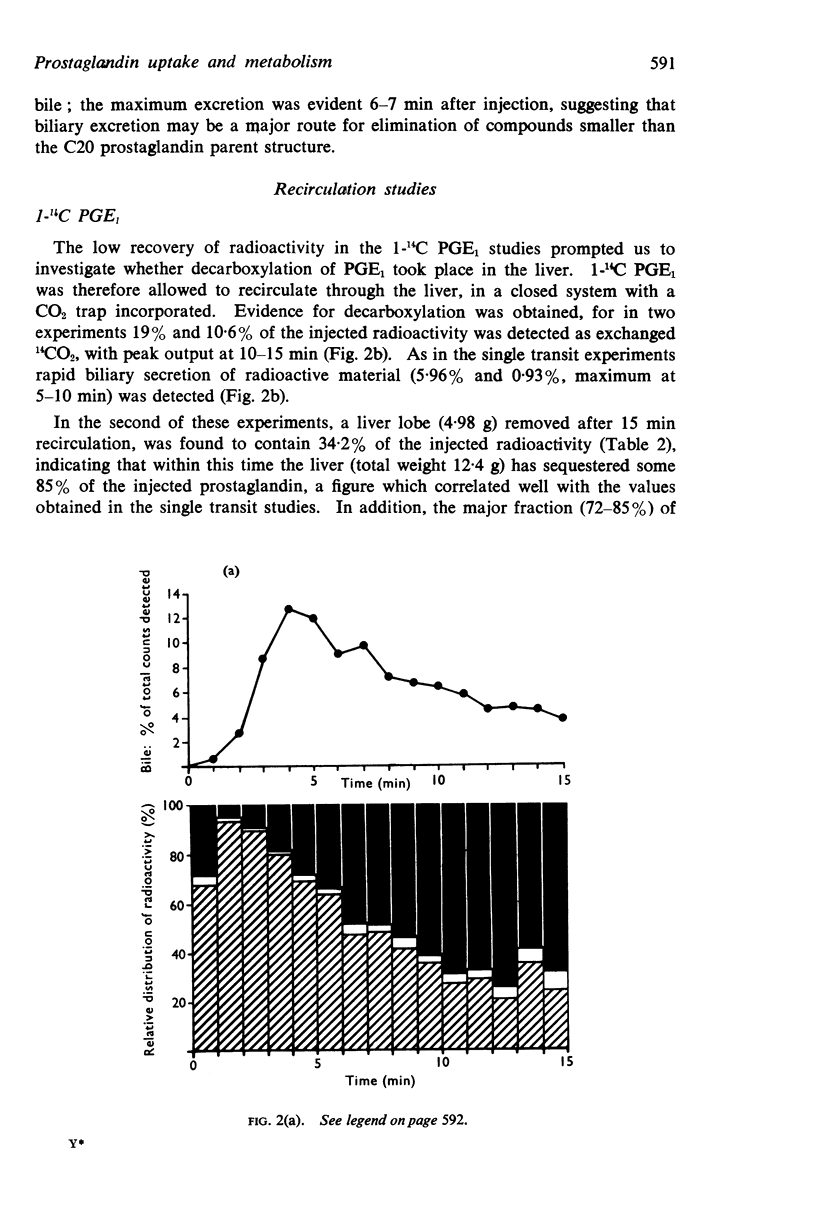
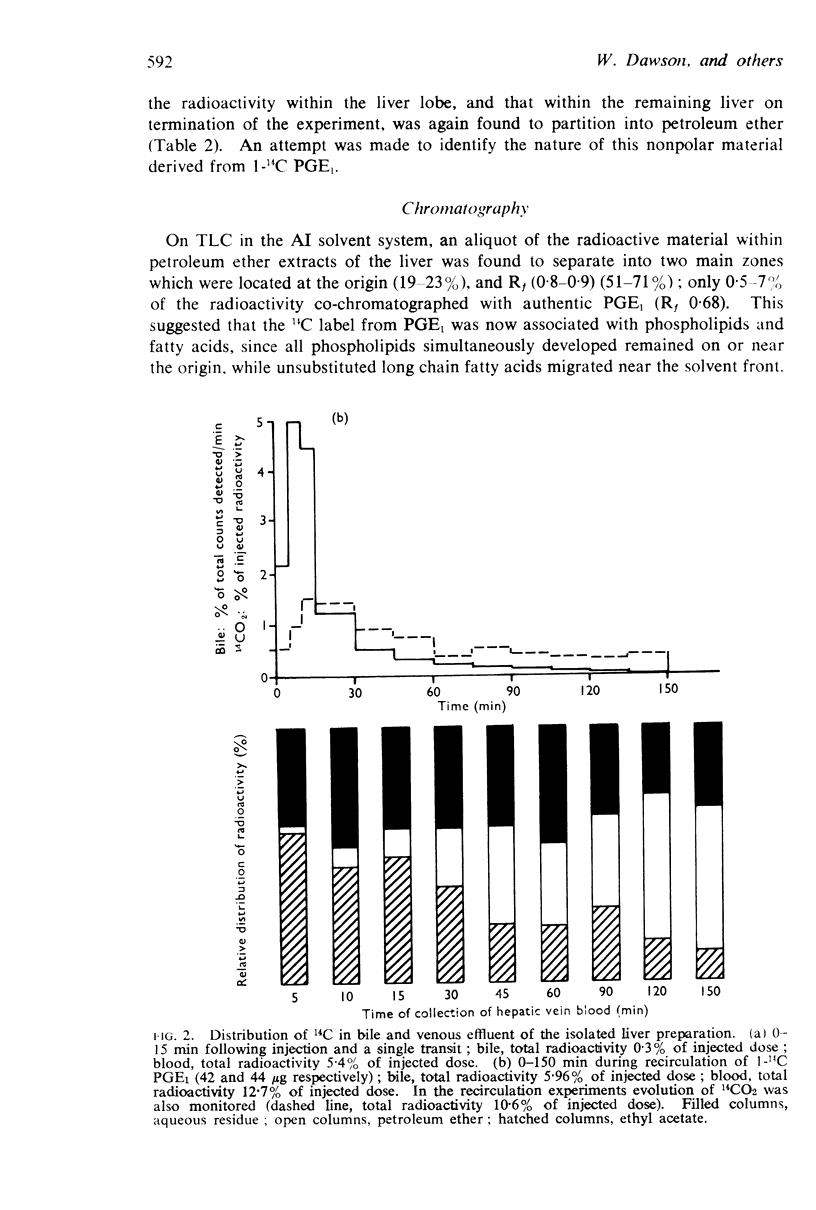
3. A single injection of 1-14C PGE1 indicated that the liver could efficiently remove 89-95% of circulating PGE1 on a single passage. Biliary excretion was excluded as a major route for elimination of unchanged PGE1, because only 0·3-0·8% of the injected radioactivity was detected in the bile. During recirculation of 1-14C PGE1, 11-19% of the injected radioactivity was detected as exchanged 14CO2. The radioactivity detected within liver was identified with further fragments resulting from decarboxylation of PGE1, which were incorporated into fatty acids and then phospholipids.
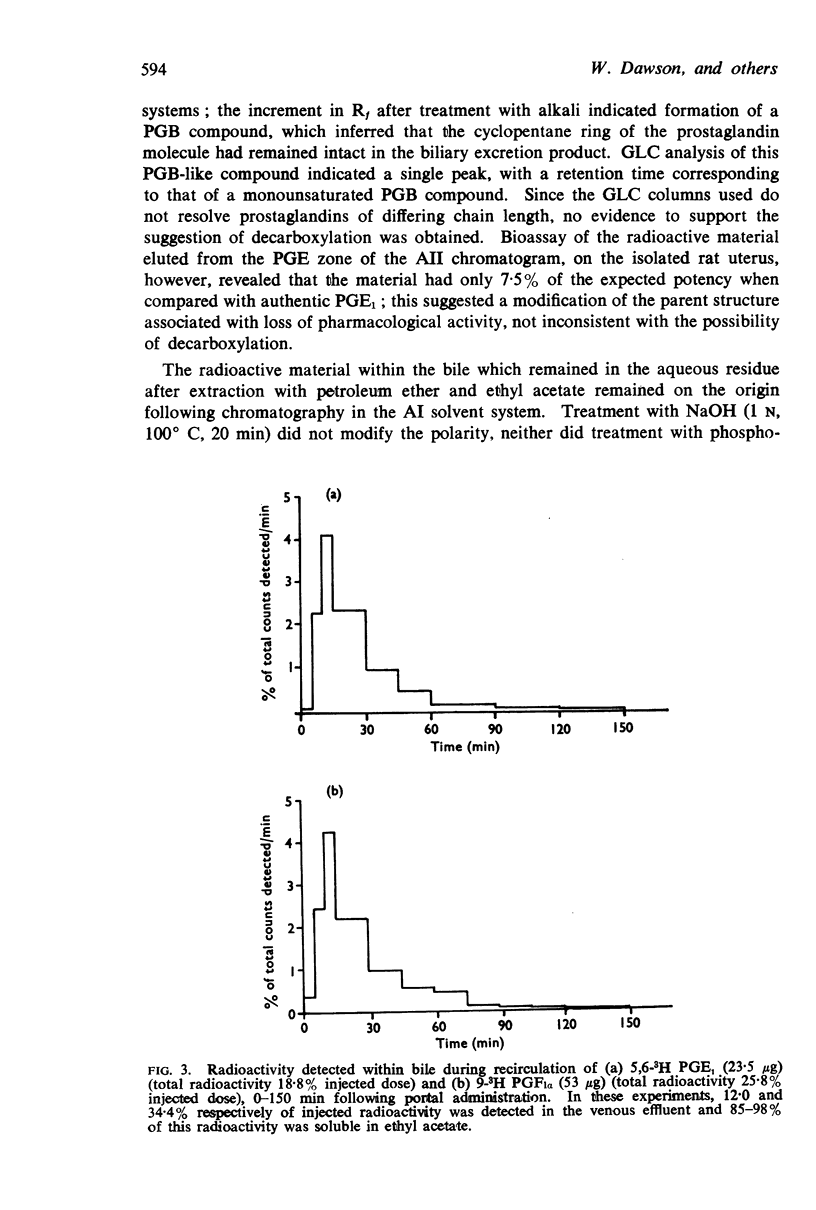
4. Studies with 5,6-3H PGE1, and comparison with the results obtained using 1-14C PGE1, revealed a 30-fold increase in the percentage of radioactivity excreted into the bile, suggesting that biliary excretion may be a major route for elimination of compounds smaller than C20 prostaglandin. Evidence that the cyclopentane ring was intact was inferred by formation of a PGB compound on treatment with alkali; similar biliary excretion of 9-3H PGF1α also occurred. In addition, the increased radioactivity detected within the liver (37%) and blood (43%) after a single injection of 5,6-3H PGE1 had the solvent partition and thin-layer chromatography properties of PGE1, but were associated with a less polar compound smaller than the C20 parent structure.
5. These results indicate rapid uptake of circulating prostaglandins by the rat liver. Decarboxylation of prostaglandins results in pharmacological inactivation. The products are excreted into the bile and venous effluent. These processes would curtail the duration of effects following prostaglandin injection.In addition, we infer from these results that any physiological action of these ubiquitous endogenous substances is likely to be localized within their tissue of origin.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Dawson W., Grasso P., Golberg L. Lysosomal changes associated with hyperoxia in the isolated perfused rat liver. Exp Mol Pathol. 1968 Jun;8(3):370–387. doi: 10.1016/s0014-4800(68)80006-1. [DOI] [PubMed] [Google Scholar]
- Anggård E., Matschinsky F. M., Samuelsson B. Prostaglandins: enzymatic analysis. Science. 1969 Jan 31;163(3866):479–480. doi: 10.1126/science.163.3866.479. [DOI] [PubMed] [Google Scholar]
- Anggård E., Matschinsky F., Samuelsson B. Enzymatic assay of the prostaglandins. Br J Pharmacol. 1968 Sep;34(1):190P–190P. [PMC free article] [PubMed] [Google Scholar]
- Anggård E. The biological activities of three metabolites of prostaglandin E 1. Acta Physiol Scand. 1966 Apr;66(4):509–510. doi: 10.1111/j.1748-1716.1966.tb03231.x. [DOI] [PubMed] [Google Scholar]
- Bergström S., Carlson L. A., Weeks J. R. The prostaglandins: a family of biologically active lipids. Pharmacol Rev. 1968 Mar;20(1):1–48. [PubMed] [Google Scholar]
- COCEANI F., WOLFE L. S. PROSTAGLANDINS IN BRAIN AND THE RELEASE OF PROSTAGLANDIN-LIKE COMPOUNDS FROM THE CAT CEREBELLAR CORTEX. Can J Physiol Pharmacol. 1965 May;43:445–450. doi: 10.1139/y65-045. [DOI] [PubMed] [Google Scholar]
- Davies B. N., Horton E. W., Withrington P. G. The occurrence of prostaglandin E2 in splenic venous blood of the dog following splenic nerve stimulation. Br J Pharmacol Chemother. 1968 Jan;32(1):127–135. doi: 10.1111/j.1476-5381.1968.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W., Ramwell P. W., Shaw J. Metabolism of prostaglandins by the rat isolated liver. Br J Pharmacol. 1968 Nov;34(3):668P–669P. [PMC free article] [PubMed] [Google Scholar]
- Edwards W. G., Jr, Strong C. G., Hunt J. C. A vasodepressor lipid resembling prostaglandin E2 (PGE2) in the renal venous blood of hypertensive patients. J Lab Clin Med. 1969 Sep;74(3):389–399. [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- GREEN K., SAMUELSSON B. PROSTAGLANDINS AND RELATED FACTORS: XIX. THIN-LAYER CHROMATOGRAPHY OF PROSTAGLANDINS. J Lipid Res. 1964 Jan;5:117–120. [PubMed] [Google Scholar]
- Granström E., Samuelsson B. The structure of the main urinary metabolite of prostaglandin F2-alpha in the guinea pig. Eur J Biochem. 1969 Oct;10(3):411–418. doi: 10.1111/j.1432-1033.1969.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Hamberg M. Metabolism of prostaglandins in rat liver mitochondria. Eur J Biochem. 1968 Oct 17;6(1):135–146. doi: 10.1111/j.1432-1033.1968.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. The structure of a urinary metabolite of prostaglandin E2 in the guinea pig. Biochem Biophys Res Commun. 1969 Jan 6;34(1):22–27. doi: 10.1016/0006-291x(69)90522-1. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. The structure of the major urinary metabolite of prostaglandin E2 in man. J Am Chem Soc. 1969 Apr 9;91(8):2177–2178. doi: 10.1021/ja01036a092. [DOI] [PubMed] [Google Scholar]
- Hansson E., Samuelsson B. Autoradiographic distribution studies of 3H-labeled prostaglandin E1 in mice. Prostaglandins and related factors 31. Biochim Biophys Acta. 1965 Oct 4;106(2):379–385. doi: 10.1016/0005-2760(65)90046-9. [DOI] [PubMed] [Google Scholar]
- Hissen W., Fleming J. S., Bierwagen M. E., Pindell M. H. Effect of prostaglandin E 1 on platelet aggregation in vitro and in hemorrhagic shock. Microvasc Res. 1969 Oct;1(4):374–378. doi: 10.1016/0026-2862(69)90015-6. [DOI] [PubMed] [Google Scholar]
- Holmes S. W., Horton E. W., Stewart M. J. Observations on the extraction of prostaglandins from blood. Life Sci. 1968 Apr 15;7(8):349–354. doi: 10.1016/0024-3205(68)90032-5. [DOI] [PubMed] [Google Scholar]
- Kloeze J. Relationship between chemical structure and platelet-aggregation activity of prostaglandins. Biochim Biophys Acta. 1969 Oct 28;187(3):285–292. doi: 10.1016/0005-2760(69)90001-0. [DOI] [PubMed] [Google Scholar]
- Parkinson T. M., Schneider J. C., Jr Absorption and metabolism of prostaglandin E1 by perfused rat jejunum in vitro. Biochim Biophys Acta. 1969 Jan 21;176(1):78–85. doi: 10.1016/0005-2760(69)90076-9. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969 Jul 5;223(5201):29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B. PROSTAGLANDINS AND RELATED FACTORS. 27. SYNTHESIS OF TRITIUM-LABELED PROSTAGLANDIN E1 AND STUDIES ON ITS DISTRIBUTION AND EXCRETION IN THE RAT. J Biol Chem. 1964 Dec;239:4091–4096. [PubMed] [Google Scholar]
- Shaw J. E., Ramwell P. W. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem. 1968 Apr 10;243(7):1498–1503. [PubMed] [Google Scholar]
- Shaw J. E., Ramwell P. W. Separation, identification, and estimation of prostaglandins. Methods Biochem Anal. 1969;17:325–371. doi: 10.1002/9780470110355.ch8. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. The release and assay of hormones in the circulation. Sci Basis Med Annu Rev. 1968:336–358. [PubMed] [Google Scholar]
- Williams E. D., Karim S. M., Sandler M. Prostaglandin secretion by medullary carcinoma of the thyroid. A possible cause of the associated idarrhoea. Lancet. 1968 Jan 6;1(7532):22–23. doi: 10.1016/s0140-6736(68)90010-x. [DOI] [PubMed] [Google Scholar]


