Abstract
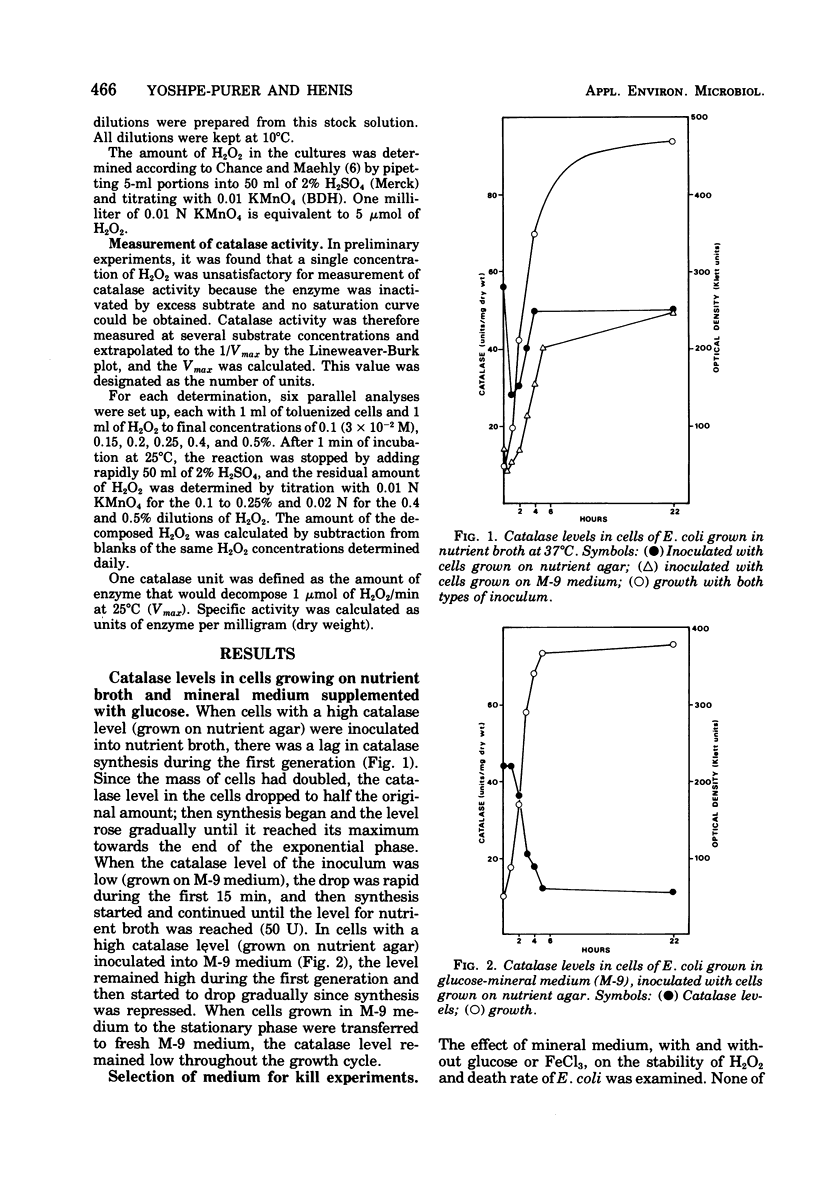
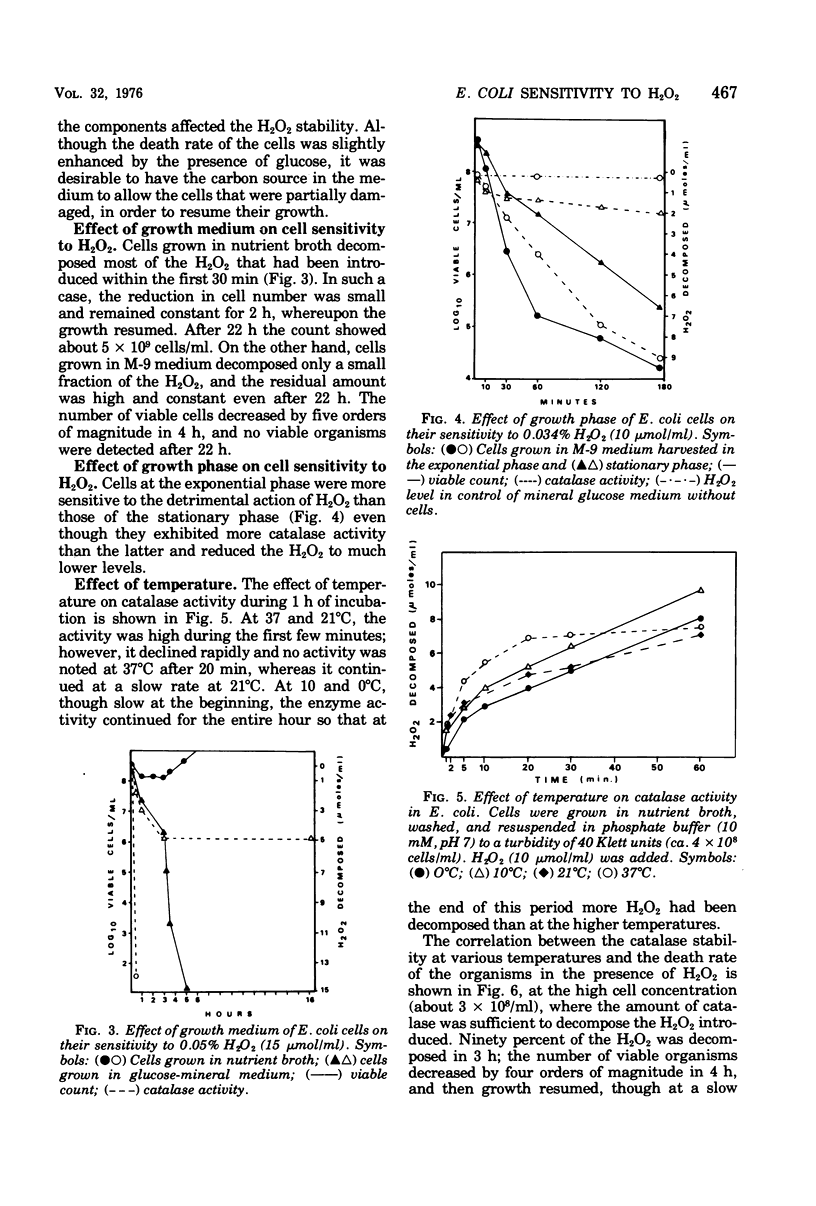
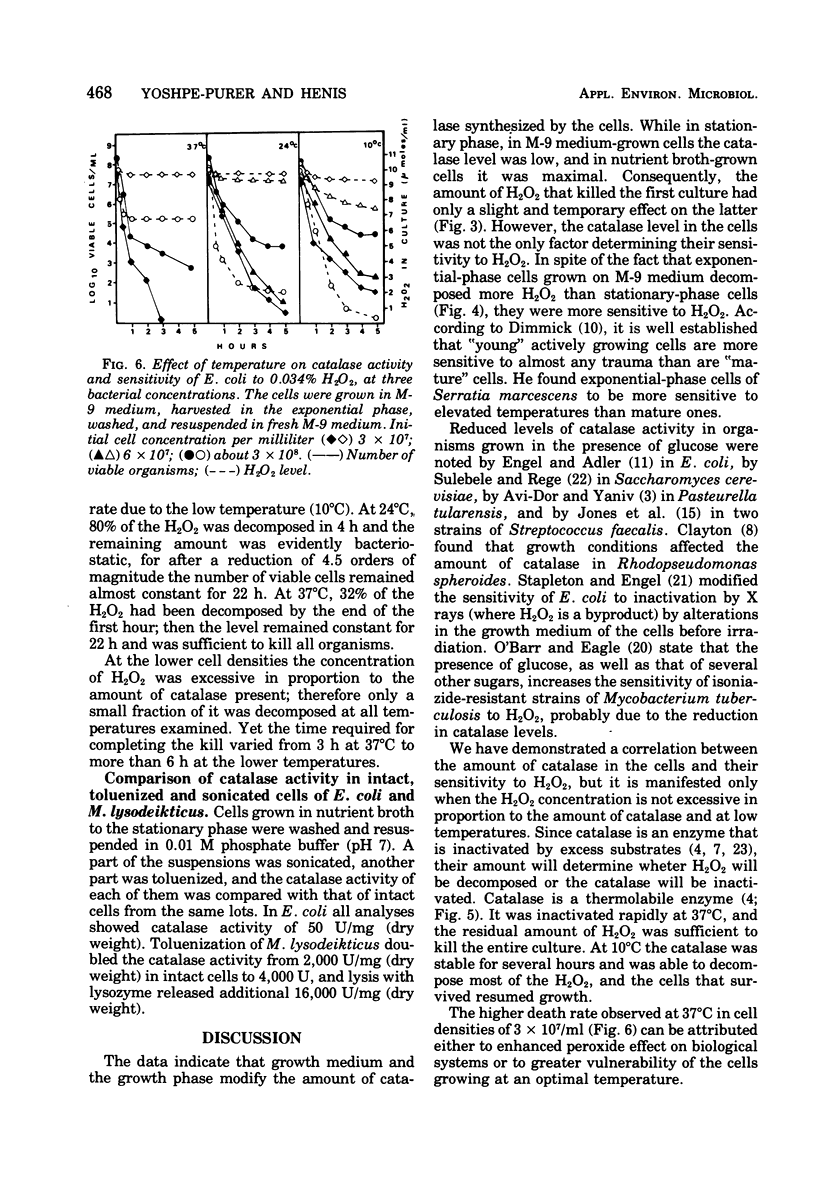
Composition of the culture medium, growth phase, and temperature play important roles in the sensitivity of Escherichia coli to H2O2. The medium and growth phase affected the sensitivity of the cells to H2O2 by modifying the amount of catalase synthesized by them, whereas the effect of temperature was due to the thermolability of the enzyme. Since catalase is unstable in the presence of its substrate, the correlation between the catalase level in the cells and their sensitivity to H2O2 could be observed only when the H2O2 concentration was not excessive in proportion to the amount of catalase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNEAR D. I., DORMAN D. C. Hydrogen peroxide accumulation during growth of the pneumococcus. Aust J Exp Biol Med Sci. 1952 Apr;30(2):191–195. doi: 10.1038/icb.1952.18. [DOI] [PubMed] [Google Scholar]
- AVI-DOR Y., YANIV H. The activity of catalase in Pasteurella tularensis. J Bacteriol. 1952 Jun;63(6):751–757. doi: 10.1128/jb.63.6.751-757.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin V. M., Olson N. F. Influence of catalase activity on resistance of coagulase-positive staphylococci to hydrogen peroxide. Appl Microbiol. 1968 Feb;16(2):267–270. doi: 10.1128/am.16.2.267-270.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. Permeability barriers and the assay of catalase in intact cells. Biochim Biophys Acta. 1959 Nov;36:35–39. doi: 10.1016/0006-3002(59)90066-6. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. The induced synthesis of catalase in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1960 Jan 29;37:503–512. doi: 10.1016/0006-3002(60)90507-2. [DOI] [PubMed] [Google Scholar]
- Campbell J. E., Dimmick R. L. Effect of 3 percent hydrogen peroxide on the viability of Serratia marcescens. J Bacteriol. 1966 Mar;91(3):925–929. doi: 10.1128/jb.91.3.925-929.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- DIMMICK R. L. RHYTHMIC RESPONSE OF SERRATIA MARCESCENS TO ELEVATED TEMPERATURE. J Bacteriol. 1965 Mar;89:791–798. doi: 10.1128/jb.89.3.791-798.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGEL M. S., ADLER H. I. Catalase activity, sensitivity to hydrogen peroxide, and radiation response in the genus Escherichia. Radiat Res. 1961 Sep;15:269–275. [PubMed] [Google Scholar]
- FAINE S. Catalase activity in pathogenic Leptospira. J Gen Microbiol. 1960 Feb;22:1–9. doi: 10.1099/00221287-22-1-1. [DOI] [PubMed] [Google Scholar]
- FEW A. V., FRASER M. J., GILBY A. R. The intracellular catalase of Micrococcus lysodeikticus. Biochim Biophys Acta. 1957 May;24(2):306–314. doi: 10.1016/0006-3002(57)90199-3. [DOI] [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES D., DEIBEL R. H., NIVEN C. F., Jr CATALASE ACTIVITY OF TWO STREPTOCOCCUS FAECALIS STRAINS AND ITS ENHANCEMENT BY AEROBIOSIS AND ADDED CATIONS. J Bacteriol. 1964 Sep;88:602–610. doi: 10.1128/jb.88.3.602-610.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low I. E., Eaton M. D., Proctor P. Relation of catalase to substrate utilization by Mycoplasma pneumoniae. J Bacteriol. 1968 Apr;95(4):1425–1430. doi: 10.1128/jb.95.4.1425-1430.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Barr T. P., Eagle M. C. Interaction of hydrogen peroxide with carbohydrates and polyhydric alcohols in the growth suppression of isoniazid-resistant Mycobacterium tuberculosis. Am Rev Respir Dis. 1967 Oct;96(4):740–744. doi: 10.1164/arrd.1967.96.4.740. [DOI] [PubMed] [Google Scholar]
- Stapleton G. E., Engel M. S. CULTURAL CONDITIONS AS DETERMINANTS OF SENSITIVITY OF ESCHERICHIA COLI TO DAMAGING AGENTS. J Bacteriol. 1960 Oct;80(4):544–551. doi: 10.1128/jb.80.4.544-551.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulebele G. A., Rege D. V. The nature of the glucose effect on the induced synthesis of catalase in Saccharomyces cerevisiae. Enzymologia. 1968 Dec 31;35(6):321–334. [PubMed] [Google Scholar]
- WEIBULL C., HAMMARBERG K. Catalase activity of Proteus L forms and normal Proteus bacteria. J Bacteriol. 1963 Feb;85:498–498. doi: 10.1128/jb.85.2.498-498.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


