Abstract
1. The antagonistic effect of thioglycerol against oxytocin and an analogue of oxytocin not containing a disulphide bridge (desamino-1-carba-oxytocin) has been compared in the rat isolated depolarized uterus.
2. Thioglycerol clearly differentiated between the two compounds, 40 mM producing a dose ratio of 12 with oxytocin but only 1·5 with the carba compound.
3. It was confirmed that thioglycerol produces no appreciable destruction of oxytocin in vitro.
4. The mode of action of thiols in antagonizing specifically S-S polypeptides is discussed. It is concluded that they probably do not produce their effect either by inactivating receptors or by inactivating S-S polypeptides in solution. A possible mechanism is that thiols potentiate the destruction of S-S polypeptides at the receptor site.
Full text
PDF

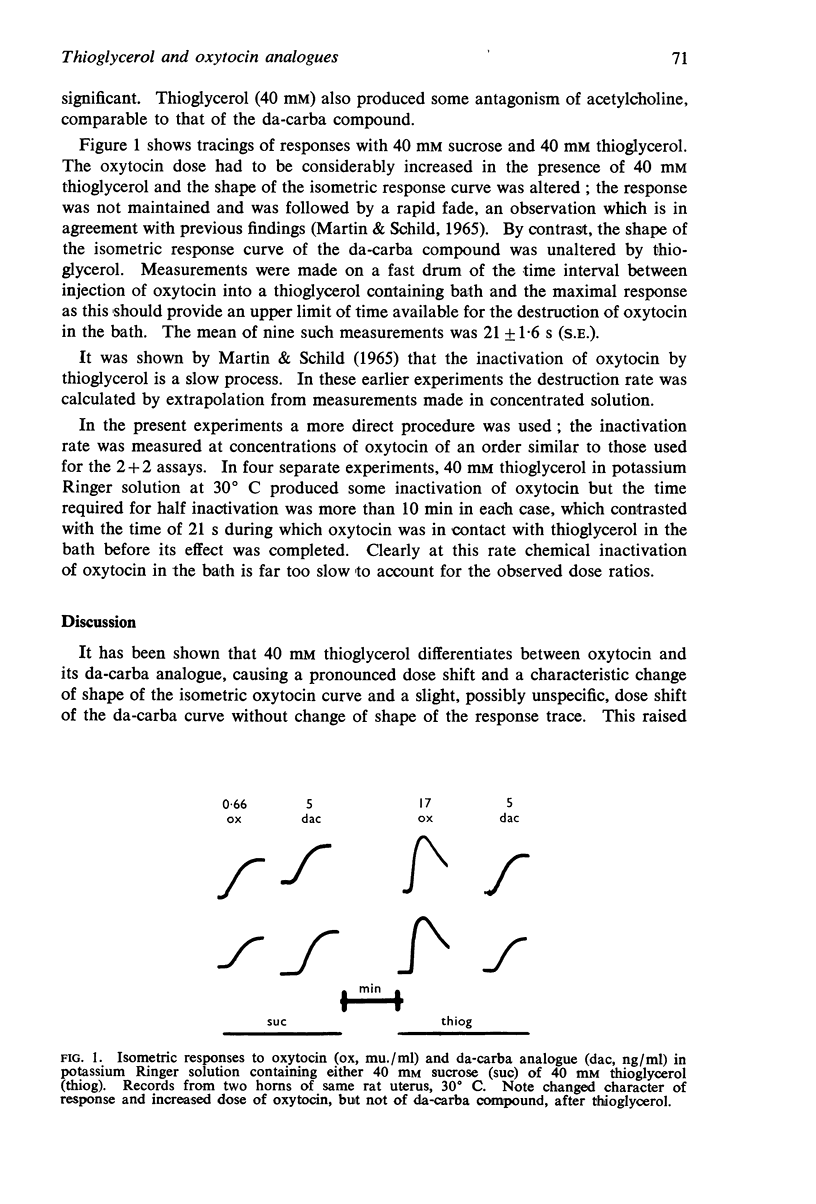

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUDRAIN L., CLAUSER H. [Mechanism of the inactivation of oxytocin by uterine tissue]. Biochim Biophys Acta. 1960 Mar 11;38:494–501. doi: 10.1016/0006-3002(60)91284-1. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Schild H. O. The antagonism of disulphide polypeptides by thiols. Br J Pharmacol Chemother. 1965 Oct;25(2):418–431. doi: 10.1111/j.1476-5381.1965.tb02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H. O. The effect of metals on the S-S polypeptide receptor in depolarized rat uterus. Br J Pharmacol. 1969 Jun;36(2):329–349. doi: 10.1111/j.1476-5381.1969.tb09509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


