Abstract
1. Skins of the lizard Anolis carolinensis darken in vitro in response to melanophore stimulating hormone (MSH), a peptide hormone, as well as to catecholamines. These hormones darken Anolis skins by dispersing the sub-cellular organelle, the melanosome, out into the dendritic processes of the dermal melanophores.
2. Dibutyryl cyclic AMP and methylxanthines also darken skins. In addition, methylxanthines are synergistic with both catecholamines and MSH in causing skin darkening. These data suggest that the dispersion of melanosomes within melanophores in response to both MSH and catecholamines is mediated by cyclic AMP.
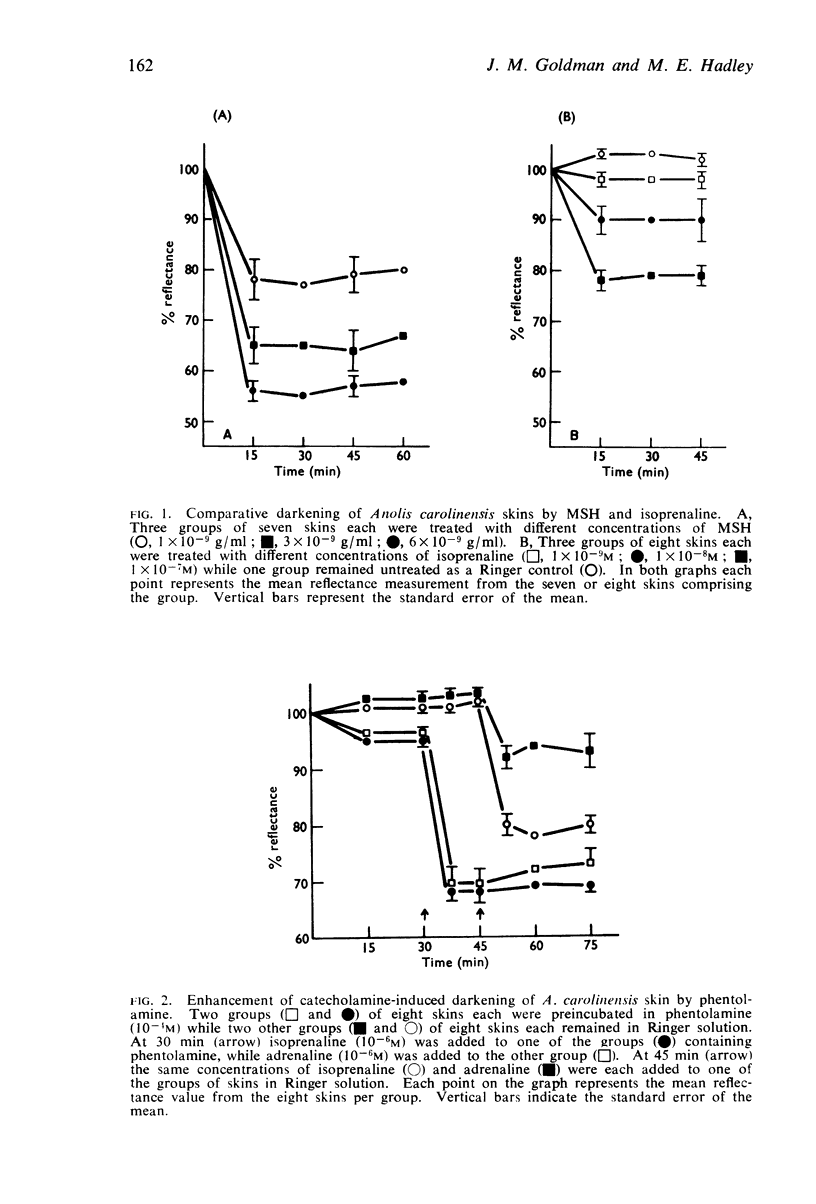
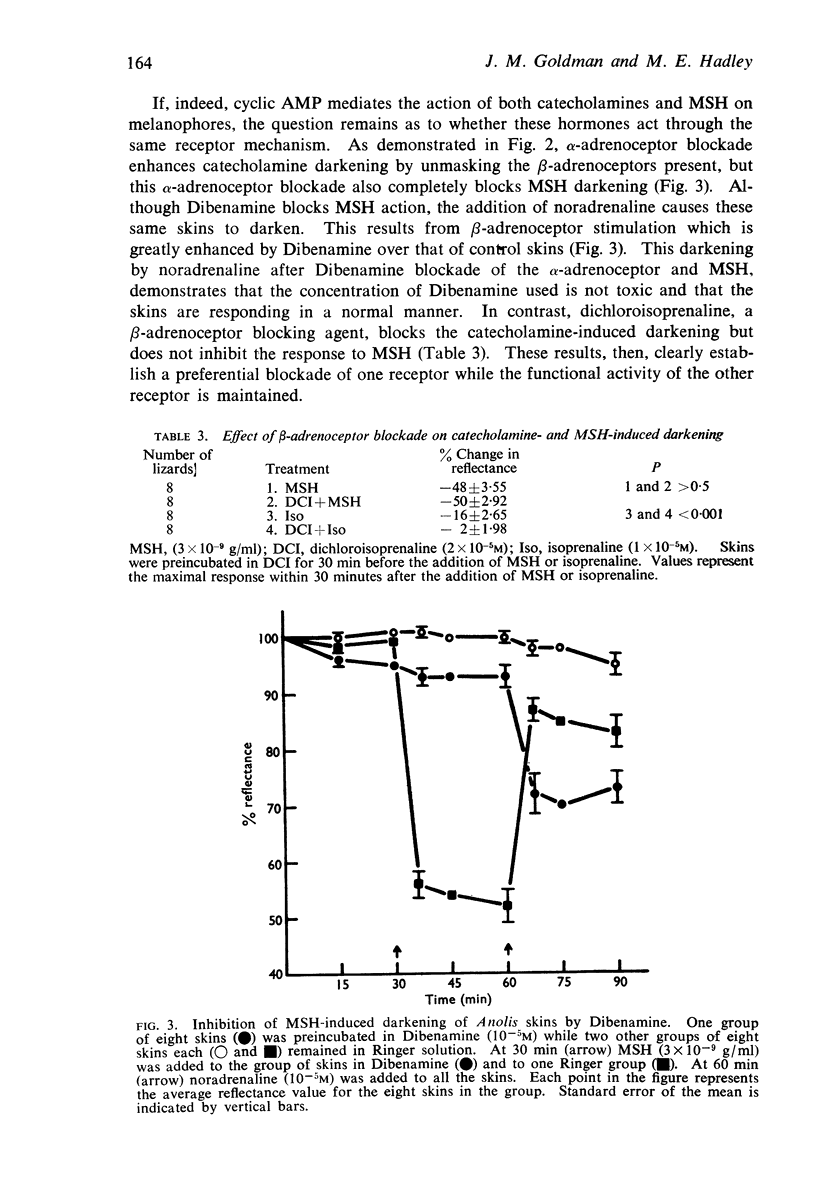
3. α-Adrenoceptor blocking agents inhibit MSH-induced darkening but potentiate catecholamine-induced darkening. β-Adrenoceptor blocking agents, in contrast, inhibit catecholamine-induced darkening but have no effect on MSH-induced darkening. This selective blockade of one receptor while the functional integrity of the other receptor is maintained suggests that MSH and catecholamines increase cyclic AMP levels through different receptors.
4. Catecholamines exert their action through β-adrenoceptors; MSH darkens skins through what appears to be a component of the α-adrenoceptor. β-Adrenoceptor stimulation may stimulate adenyl cyclase to increase cyclic AMP levels whereas MSH may inhibit cyclic AMP phosphodiesterase thereby preventing cyclic AMP breakdown.
Full text
PDF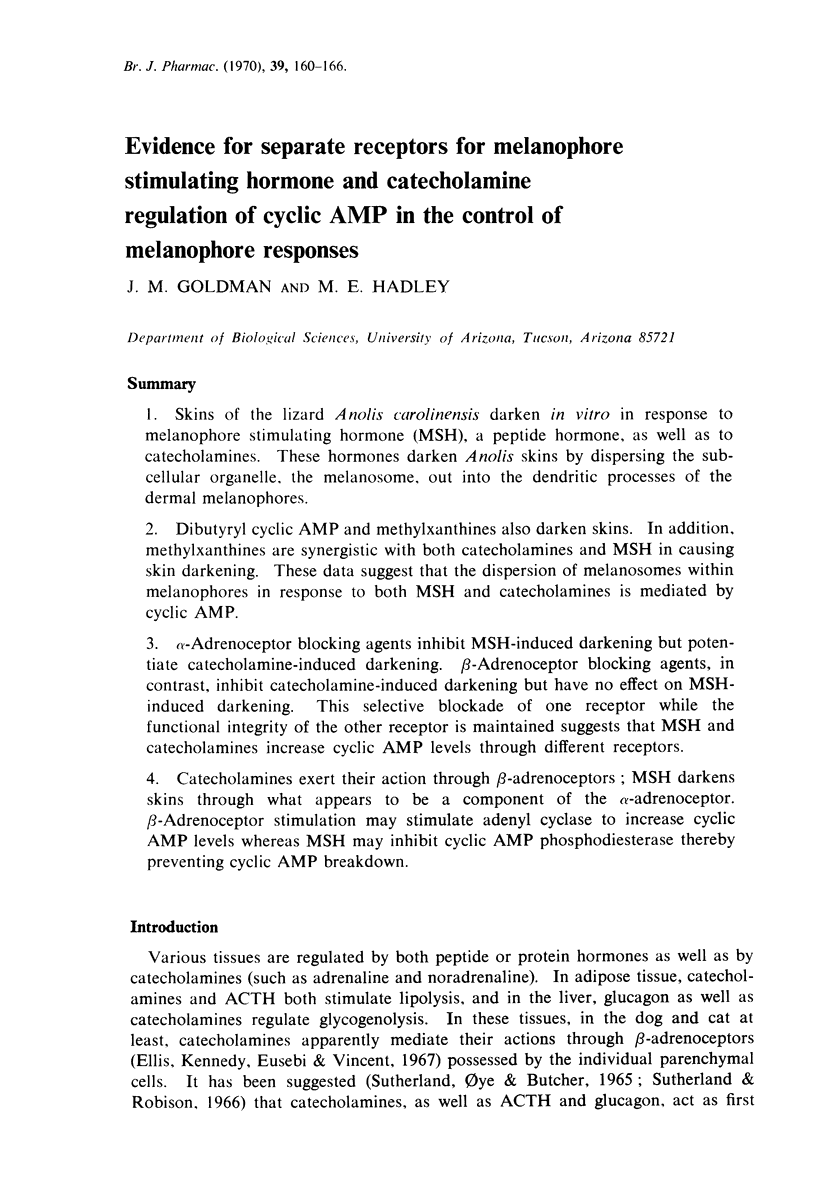




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Bitensky M. W., Russell V., Robertson W. Evidence for separate epinephrine and glucagon responsive adenyl cyclase systems in rat liver. Biochem Biophys Res Commun. 1968 Jun 10;31(5):706–712. doi: 10.1016/0006-291x(68)90619-0. [DOI] [PubMed] [Google Scholar]
- Ellis S., Kennedy B. L., Eusebi A. J., Vincent N. H. Autonomic control of metabolism. Ann N Y Acad Sci. 1967 Feb 10;139(3):826–832. doi: 10.1111/j.1749-6632.1967.tb41252.x. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Adrenergic blockade of hormone-induced lipolysis in isolated fat cells. Ann N Y Acad Sci. 1967 Feb 10;139(3):879–890. doi: 10.1111/j.1749-6632.1967.tb41257.x. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Galton D. J., Kovacev V. P. Effect of drugs on the lipolytic action of hormones in isolated fat cells. Mol Pharmacol. 1966 May;2(3):237–247. [PubMed] [Google Scholar]
- Goldman J. M., Hadley M. E. In vitro demonstration of adrenergic receptors controlling melanophore responses of the lizard, Anolis carolinensis. J Pharmacol Exp Ther. 1969 Mar;166(1):1–7. [PubMed] [Google Scholar]
- Goldman J. M., Hadley M. E. The beta adrenergic receptor and cyclic 3',5'-adenosine monophosphate: possible roles in the regulation of melanophore responses of the spadefoot toad, Scaphiopus couchi. Gen Comp Endocrinol. 1969 Aug;13(1):151–163. doi: 10.1016/0016-6480(69)90232-9. [DOI] [PubMed] [Google Scholar]
- Hadley M. E., Goldman J. M. Physiological color changes in reptiles. Am Zool. 1969 May;9(2):489–504. doi: 10.1093/icb/9.2.489. [DOI] [PubMed] [Google Scholar]
- Laraia P. J., Craig R. J., Reddy W. J. Glucagon: effect on adenosine 3', 5'-monophosphate in the rat heart. Am J Physiol. 1968 Oct;215(4):968–970. doi: 10.1152/ajplegacy.1968.215.4.968. [DOI] [PubMed] [Google Scholar]
- Lech J. J., Calvert D. N. Some evidence for differentiation of ACTH and norepinephrine lipolytic receptors in adipose cells. Life Sci. 1967 Apr 15;6(8):833–844. doi: 10.1016/0024-3205(67)90286-x. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Activation of adenyl cyclase by glucagon in cat and human heart. Circ Res. 1969 Feb;24(2):151–156. doi: 10.1161/01.res.24.2.151. [DOI] [PubMed] [Google Scholar]
- Moran N. C. Pharmacological characterization of adrenergic receptors. Pharmacol Rev. 1966 Mar;18(1):503–512. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- SHIZUME K., LERNER A. B., FITZPATRICK T. B. In vitro bioassay for the melanocyte stimulating hormone. Endocrinology. 1954 May;54(5):553–560. doi: 10.1210/endo-54-5-553. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., OYE I., BUTCHER R. W. THE ACTION OF EPINEPHRINE AND THE ROLE OF THE ADENYL CYCLASE SYSTEM IN HORMONE ACTION. Recent Prog Horm Res. 1965;21:623–646. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958 Jun;232(2):1077–1091. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Wenke M., Lincová D., Cepelík J., Cernohorský M., Hynie S. Some aspects of the action of beta adrenergic blocking drugs on adrenergic lipid mobilization. Ann N Y Acad Sci. 1967 Feb 10;139(3):860–878. doi: 10.1111/j.1749-6632.1967.tb41256.x. [DOI] [PubMed] [Google Scholar]


