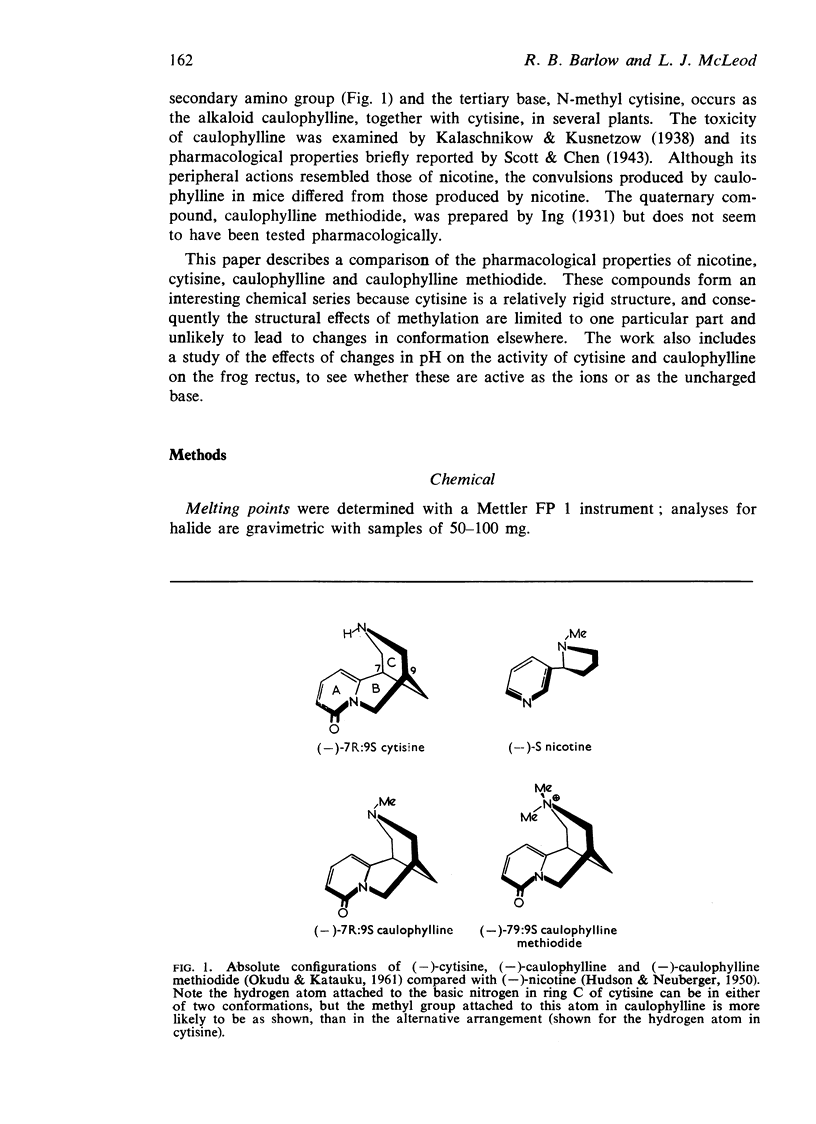
Abstract
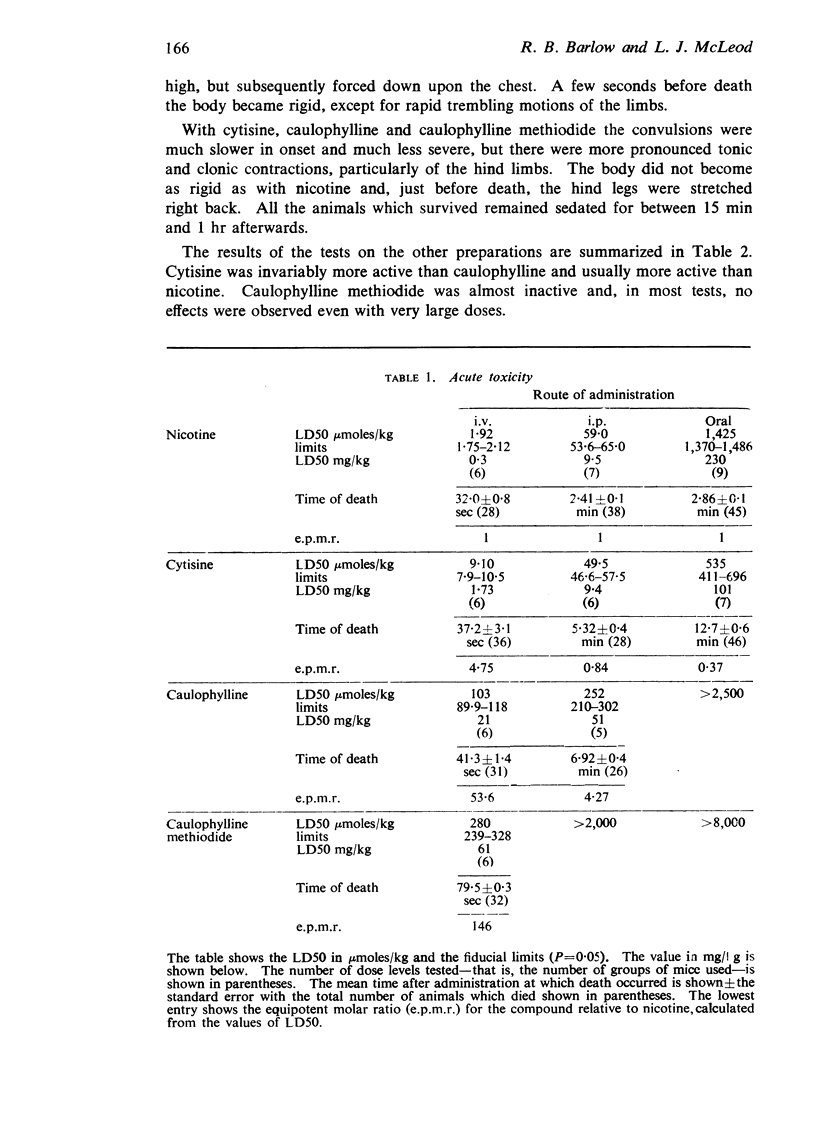
1. In mice cytisine hydrochloride is less toxic intravenously than nicotine hydrogen tartrate, but more toxic by intraperitoneal or oral administration. Compared with cytisine, caulophylline hydrogen iodide is one-fifth to one-tenth as toxic and caulophylline methiodide is less than one-thirtieth as toxic.
2. The surprising low oral toxicity of cytisine and nicotine may be ascribed to the method of administration; if the drug is placed directly in the stomach there is no possibility of absorption through buccal mucous membranes.
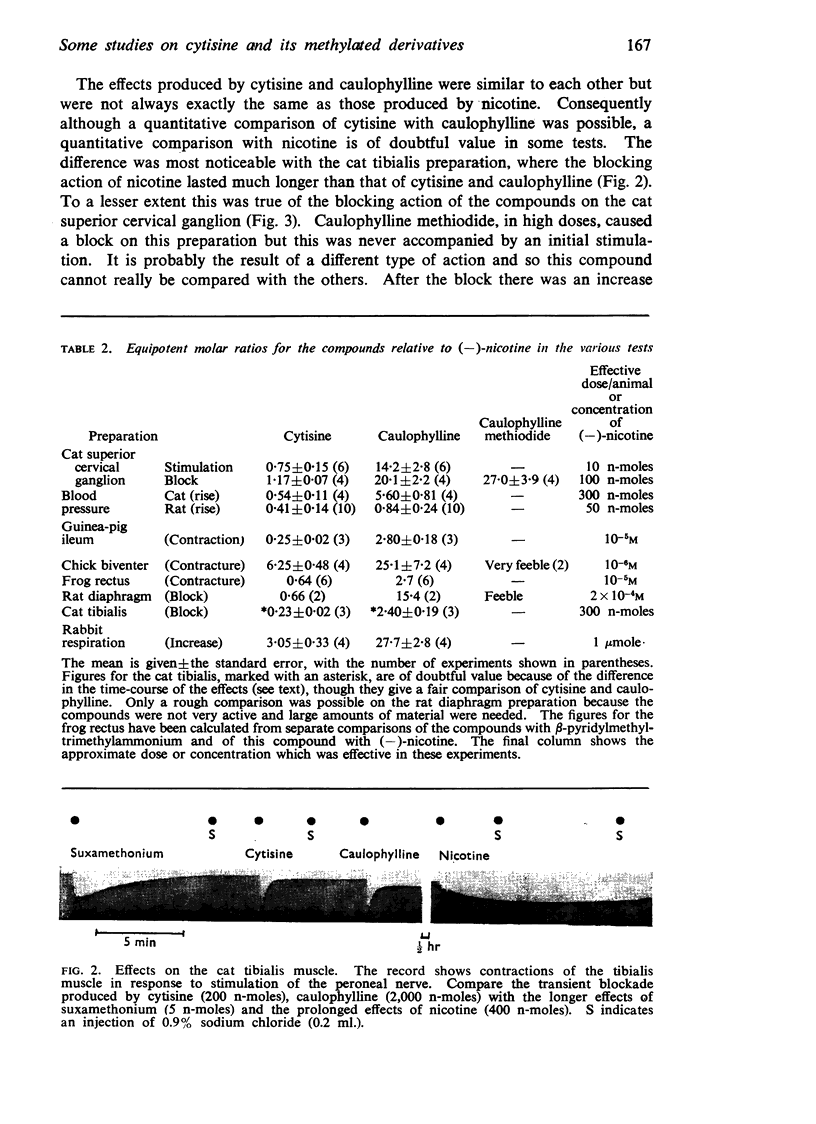
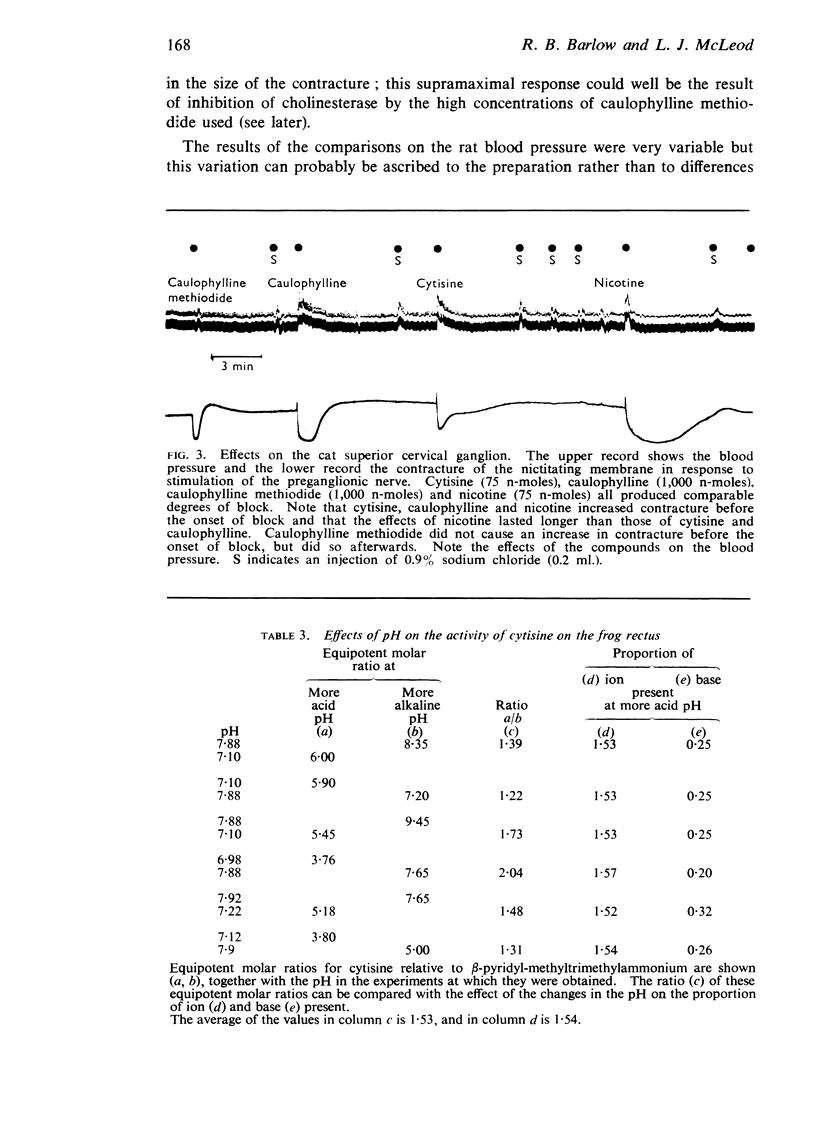
3. The peripheral effects of nicotine, cytisine and caulophylline are similar, though on some preparations those of nicotine last longer. In most tests cytisine is active in doses from a quarter to three-quarters of those of nicotine, caulophylline in doses from 10 to 20 times those of cytisine. Caulophylline methiodide is virtually inactive.
4. Cytisine and caulophylline may differ from nicotine in their central effects.
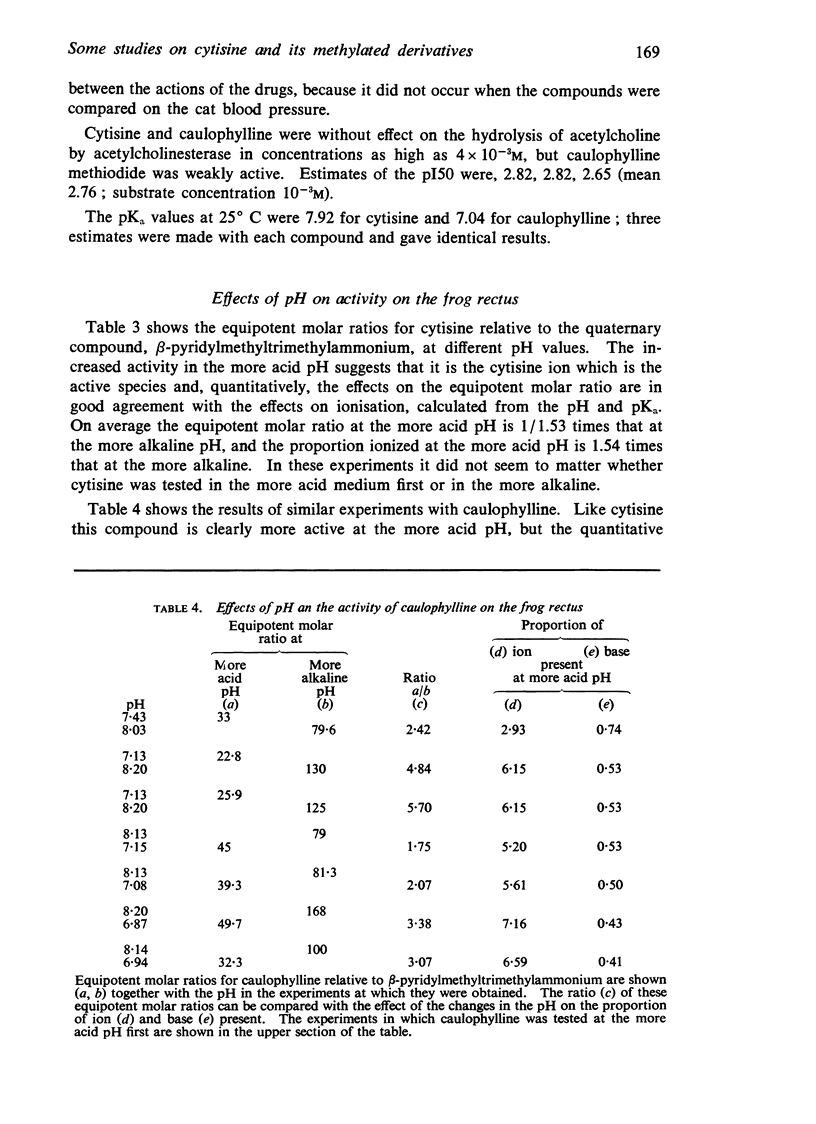
5. Cytisine and caulophylline are active as the cations. The pKa of cytisine is 7.92 and that of caulophylline is 7.04; the difference accounts, in part, for the weaker activity of caulophylline. The caulophylline ion is generally one-sixth to one-third as active as the cytisine ion.
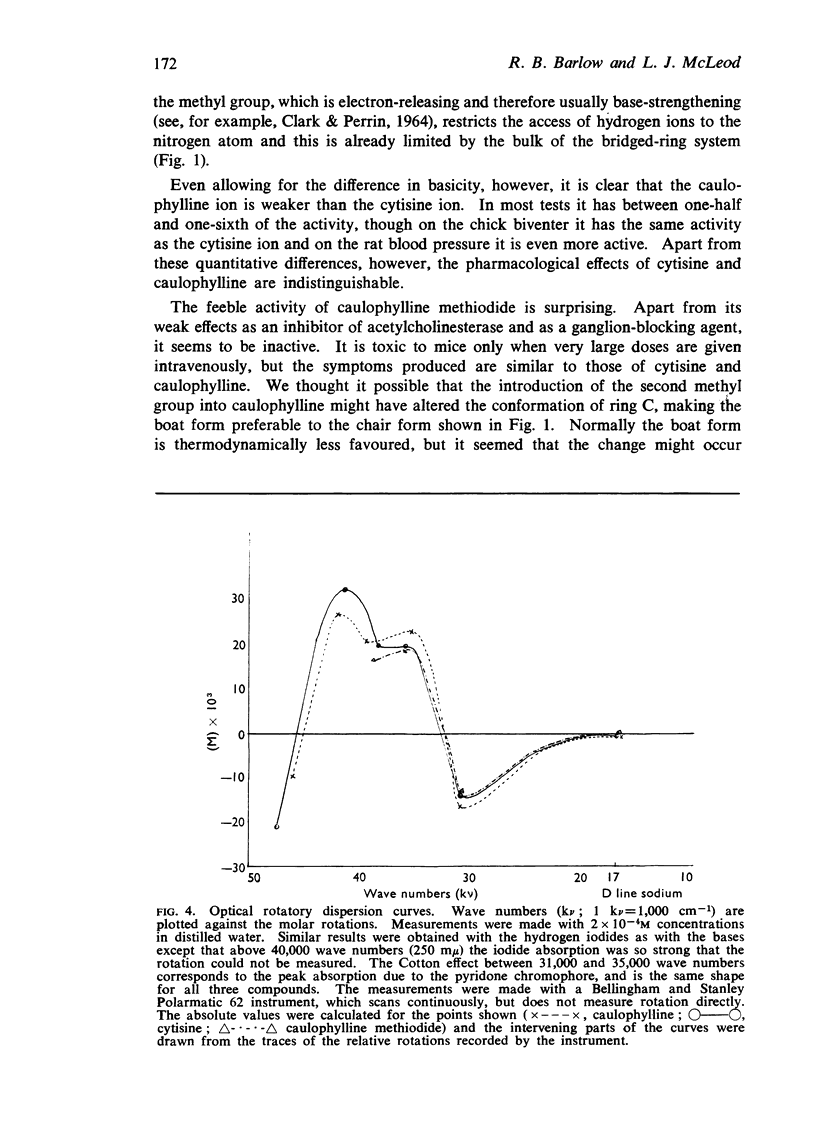
6. The introduction of the second methyl group to form the quaternary salt does not appear to cause a dramatic change in the conformation of the molecule. Caulophylline methiodide appears to be feebly active because it has feeble affinity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW R. B., ZOLLER A. SOME EFFECTS OF LONG CHAIN POLYMETHYLENE BISONIUM SALTS ON JUNCTIONAL TRANSMISSION IN THE PERIPHERAL NERVOUS SYSTEM. Br J Pharmacol Chemother. 1964 Aug;23:131–150. doi: 10.1111/j.1476-5381.1964.tb01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Scott N. C., Stephenson R. P. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967 Sep;31(1):188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum J. H. A method of recording the respiration. J Physiol. 1941 Jan 14;99(2):257–264. doi: 10.1113/jphysiol.1941.sp003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON J. T. The influence of pH on the activity of nicotine at the neuromuscular junction. Can J Biochem Physiol. 1963 Feb;41:283–289. [PubMed] [Google Scholar]
- Kier L. B. A molecular orbital calculation of the preferred conformation of nicotine. Mol Pharmacol. 1968 Jan;4(1):70–76. [PubMed] [Google Scholar]
- MITCHELL R. G. Laburnum poisoning in children; report on ten cases. Lancet. 1951 Jul 14;2(6672):57–58. doi: 10.1016/s0140-6736(51)91315-3. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., PERRY W. L. M. The relationship between depolarization and block in the cat's superior cervical ganglion. J Physiol. 1953 Jan;119(1):43–57. doi: 10.1113/jphysiol.1953.sp004827. [DOI] [PMC free article] [PubMed] [Google Scholar]