Abstract
1. Effects of cocaine on the magnitude of responses to several biologically active amines and on their rates of inactivation were studied in strips of rabbit thoracic aorta in vitro.
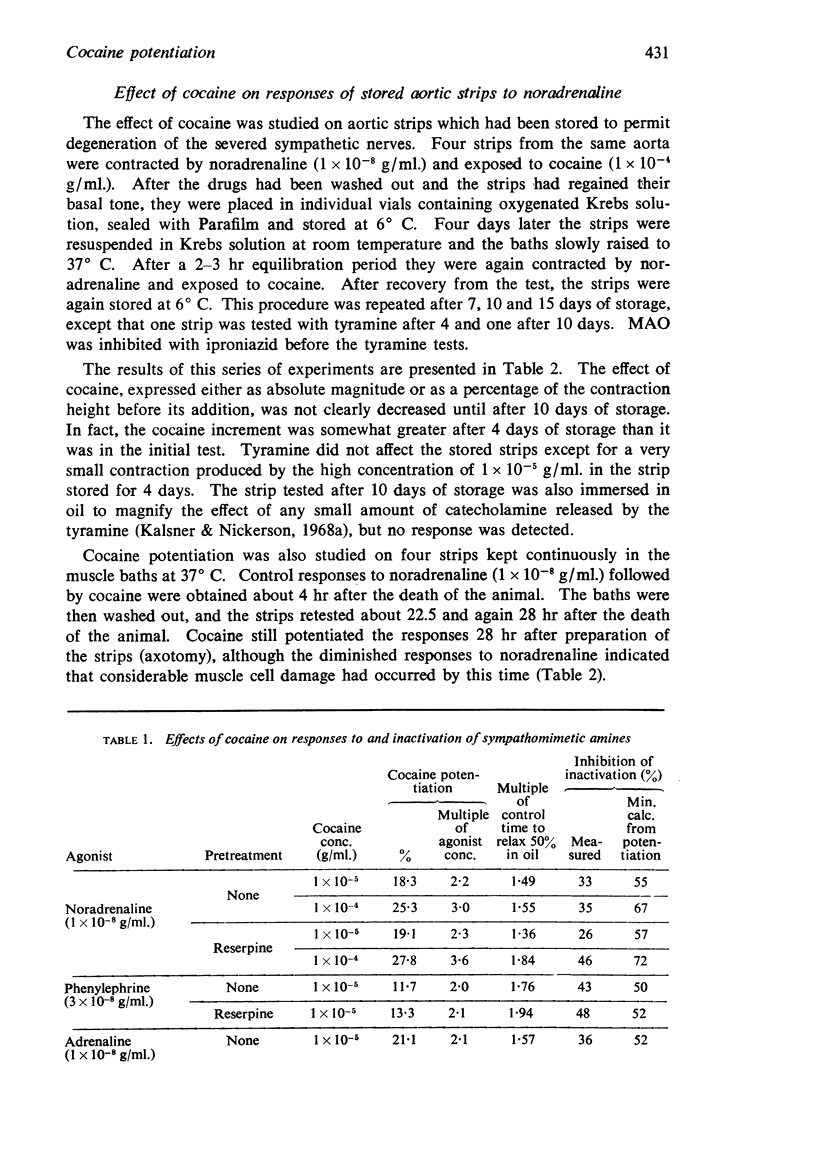
2. Although cocaine both potentiated responses to noradrenaline, adrenaline and phenylephrine and slowed their inactivation, the correlation between these two parameters under various experimental conditions was poor, and in all cases the delay in intrinsic inactivation was inadequate to account for the observed potentiation.
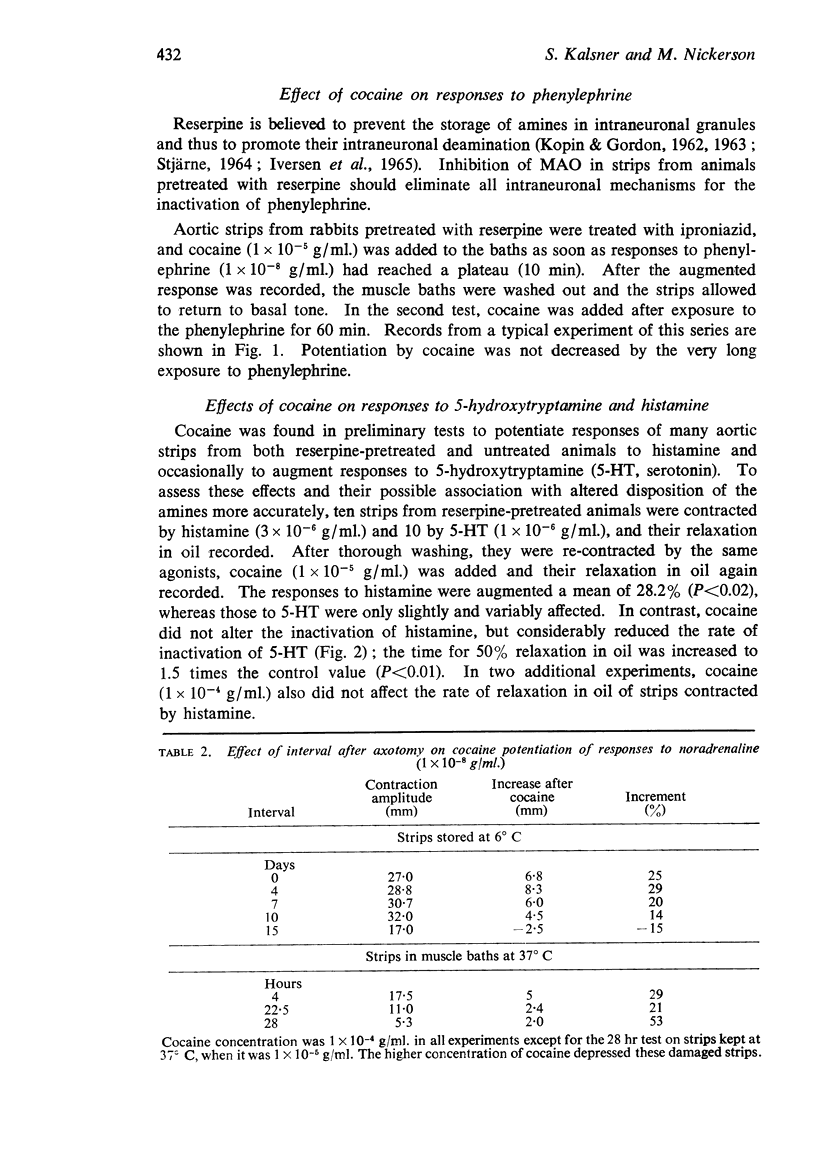
3. Potentiation of responses to noradrenaline by cocaine was little decreased in strips stored at 6° C for up to 10 days, although the response to low doses of tyramine was abolished much earlier. Similarly, cocaine clearly potentiated responses to noradrenaline for at least 28 hr at 37° C, at which time responses to noradrenaline alone were markedly decreased.
4. Cocaine potentiated responses to phenylephrine as well after 60 as after 10 min exposure to the amine in strips in which all intra-neuronal disposition of this amine had been eliminated by treatment with reserpine and iproniazid.
5. Cocaine effectively potentiated responses to histamine, but had only a slight and variable effect on those to 5-hydroxytryptamine (5-HT). It did not alter the tissue inactivation of histamine, but did significantly slow the inactivation of 5-HT.
6. Procaine slowed amine inactivation in the same way and to the same extent as did cocaine, but did not potentiate responses or affect the potentiation produced by cocaine added in its presence.
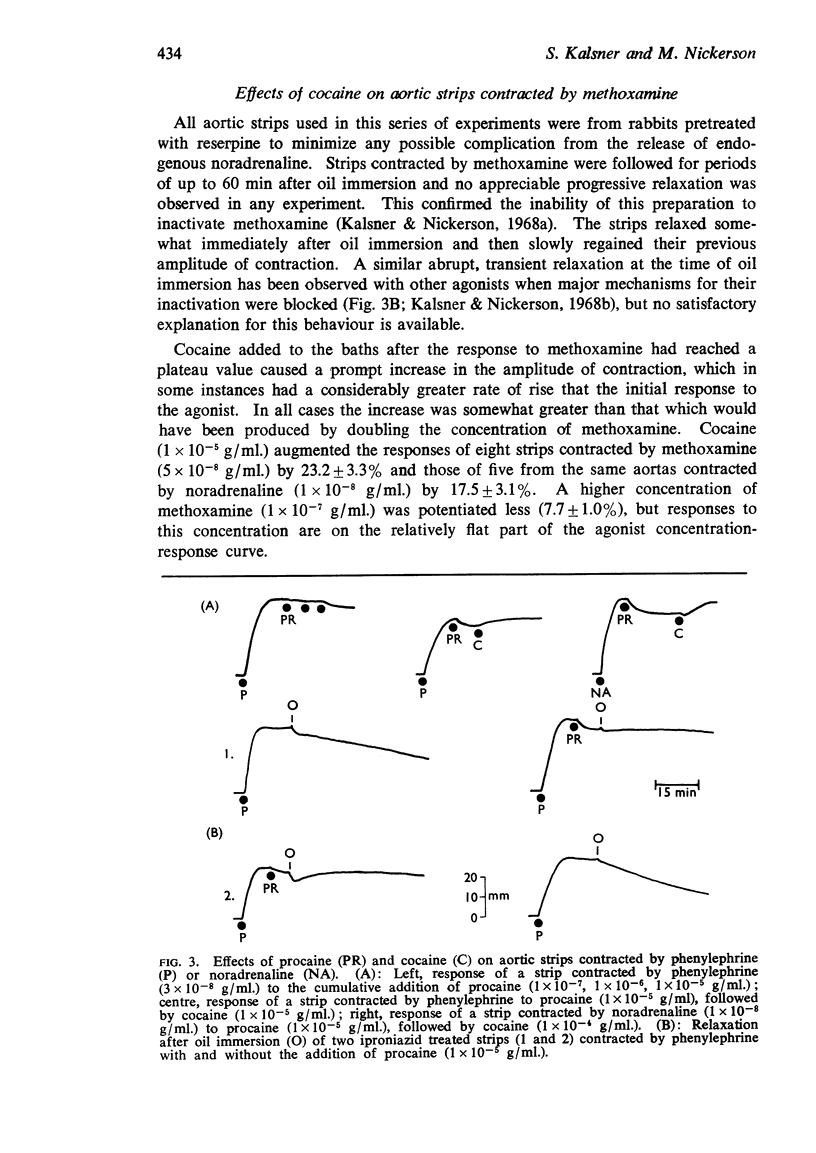
7. Cocaine potentiated responses to methoxamine to approximately the same degree as it did those to noradrenaline, although studies by the oil immersion technique clearly demonstrated that the aortic strips were entirely incapable of inactivating methoxamine.
8. The observations reported and discussed are incompatible with the hypothesis that cocaine potentiates responses to sympathomimetic amines because it prevents their inactivation by nerve uptake and storage and thus diverts larger amounts of agonist to tissue receptors. It is concluded that potentiation and inhibition of amine inactivation reflect two largely independent actions of cocaine in this vascular smooth muscle preparation, and probably in other organs, and that potentiation is a generally unreliable criterion of the blockade of processes inactivating sympathomimetic amines or of the importance of these processes in terminating the action of the amines.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., INSCOE J. K. THE UPTAKE AND BINDING OF CIRCULATING SEROTONIN AND THE EFFECT OF DRUGS. J Pharmacol Exp Ther. 1963 Aug;141:161–165. [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958 Dec 4;144(2):314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROUT J. R. Effect of inhibiting both catechol-O-methyl transferase and monoamine oxidase on cardiovascular responses to norepinephrine. Proc Soc Exp Biol Med. 1961 Nov;108:482–484. doi: 10.3181/00379727-108-26972. [DOI] [PubMed] [Google Scholar]
- DAHLSTROEM A., FUXE K., HILLARP N. A. SITE OF ACTION OF RESERPINE. Acta Pharmacol Toxicol (Copenh) 1965;22:277–292. doi: 10.1111/j.1600-0773.1965.tb01823.x. [DOI] [PubMed] [Google Scholar]
- DENGLER H. J., SPIEGEL H. E., TITUS E. O. Effects of drugs on uptake of isotopic norepinephrine by cat tissues. Nature. 1961 Aug 19;191:816–817. doi: 10.1038/191816a0. [DOI] [PubMed] [Google Scholar]
- EMMELIN N., MALM L. DEVELOPMENT OF SUPERSENSITIVITY AS DEPENDENT ON THE LENGTH OF DEGENERATING NERVE FIBRES. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:142–145. doi: 10.1113/expphysiol.1965.sp001776. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F., KIRPEKAR S. M., RIEKER M., SCHWAB A. ACTIONS AND INTERACTIONS OF NOREPINEPHRINE, TYRAMINE AND COCAINE ON AORTIC STRIPS OF RABBIT AND LEFT ATRIA OF GUINEA PIG AND CAT. J Pharmacol Exp Ther. 1963 Oct;142:39–58. [PubMed] [Google Scholar]
- FURCHGOTT R. F. The pharmacology of vascular smooth muscle. Pharmacol Rev. 1955 Jun;7(2):183–265. [PubMed] [Google Scholar]
- GRIESEMER E. C., BARSKY J., DRAGSTEDT C. A., WELLS J. A., ZELLER E. A. Potentiating effect of iproniazid on the pharmacological action of sympathomimetic amines. Proc Soc Exp Biol Med. 1953 Dec;84(3):699–701. doi: 10.3181/00379727-84-20757. [DOI] [PubMed] [Google Scholar]
- HAEFELY W., HUERLIMANN A., THOENEN H. A QUANTITATIVE STUDY OF THE EFFECT OF COCAINE ON THE RESPONSE OF THE CAT NICTITATING MEMBRANE TO NERVE STIMULATION AND TO INJECTED NORADRENALINE. Br J Pharmacol Chemother. 1964 Feb;22:5–21. doi: 10.1111/j.1476-5381.1964.tb01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTTING G., AXELROD J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature. 1961 Oct 14;192:172–173. doi: 10.1038/192172a0. [DOI] [PubMed] [Google Scholar]
- IVERSEN L. L. INHIBITION OF NORADRENALINE UPTAKE BY SYMPATHOMIMETIC AMINES. J Pharm Pharmacol. 1964 Jun;16:435–437. doi: 10.1111/j.2042-7158.1964.tb07488.x. [DOI] [PubMed] [Google Scholar]
- IVERSEN L. L. THE UPTAKE OF NORADRENALINE BY THE ISOLATED PERFUSED RAT HEART. Br J Pharmacol Chemother. 1963 Dec;21:523–537. doi: 10.1111/j.1476-5381.1963.tb02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Glowinski J., Axelrod J. The uptake and storage of H3-norepinephrine in the reserpine-pretreated rat heart. J Pharmacol Exp Ther. 1965 Nov;150(2):173–183. [PubMed] [Google Scholar]
- KOPIN I. J., GORDON E. K. Metabolism of administered and drug-released norepinephrine-7-H3 in the rat. J Pharmacol Exp Ther. 1963 May;140:207–216. [PubMed] [Google Scholar]
- KOPIN I. J., GORDON E. K. Metabolism of norepinephrine-H3 released by tyramine and reserpine. J Pharmacol Exp Ther. 1962 Dec;138:351–359. [PubMed] [Google Scholar]
- KOPIN I. J. STORAGE AND METABOLISM OF CATECHOLAMINES: THE ROLE OF MONOAMINE OXIDASE. Pharmacol Rev. 1964 Jun;16:179–191. [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. A method for the study of mechanisms of drug disposition in smooth muscle. Can J Physiol Pharmacol. 1968 Sep;46(5):719–730. doi: 10.1139/y68-113. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Disposition of phenylephrine in vascular tissue, determined by the oil-immersion technique. J Pharmacol Exp Ther. 1968 Sep;163(1):1–10. [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Effects of reserpine on the disposition of sympathomimetic amines in vascular tissue. Br J Pharmacol. 1969 Mar;35(3):394–405. doi: 10.1111/j.1476-5381.1969.tb08281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACMILLAN W. H. A hypothesis concerning the effect of cocaine on the action of sympathomimetic amines. Br J Pharmacol Chemother. 1959 Sep;14:385–391. doi: 10.1111/j.1476-5381.1959.tb00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAXWELL R. A. CONCERNING THE MECHANISM OF ACTION OF METHYLPHENIDATE ON THE RESPONSES OF RABBIT VASCULAR TISSUE TO NOREPINEPHRINE. J Pharmacol Exp Ther. 1965 Mar;147:289–297. [PubMed] [Google Scholar]
- MAXWELL R. A. CONCERNING THE MODE OF ACTION OF GUANETHIDINE AND SOME DERIVATIVES IN AUGMENTING THE VASOMOTOR ACTION OF ADRENERGIC AMINES IN VASCULAR TISSUES OF THE RABBIT. J Pharmacol Exp Ther. 1965 Jun;148:320–328. [PubMed] [Google Scholar]
- MAXWELL R. A., DANIEL A. I., SHEPPARD H., ZIMMERMAN J. H. Some interactions of guanethidine, cocaine, methylphenidate and phenylalkylamines in rabbit aortic strips. J Pharmacol Exp Ther. 1962 Jul;137:31–38. [PubMed] [Google Scholar]
- MUSCHOLL E. Effect of cocaine and related drugs on the uptake of noradrenaline by heart and spleen. Br J Pharmacol Chemother. 1961 Jun;16:352–359. doi: 10.1111/j.1476-5381.1961.tb01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmfors T., Sachs C. Direct studies on the disappearance of the transmitter and changes in the uptake-storage mechanisms of degenerating adrenergic nerves. Acta Physiol Scand. 1965 Jul;64(3):211–223. doi: 10.1111/j.1748-1716.1965.tb04171.x. [DOI] [PubMed] [Google Scholar]
- Maxwell R. A., Wastila W. B., Eckhardt S. B. Some factors determining the response of rabbit aortic strips to dl-norepinephrine-7-H3 hydrochloride and the influence of cocaine, guanethidine and methylphenidate on these factors. J Pharmacol Exp Ther. 1966 Feb;151(2):253–261. [PubMed] [Google Scholar]
- ROSELL S., KOPIN I. J., AXELROD J. FATE OF H3-NORADRENALINE IN SKELETAL MUSCLE BEFORE AND FOLLOWING SYMPATHETIC STIMULATION. Am J Physiol. 1963 Aug;205:317–321. doi: 10.1152/ajplegacy.1963.205.2.317. [DOI] [PubMed] [Google Scholar]
- THOENEN H., HUERLIMANN A., HAEFELY W. THE EFFECT OF SYMPATHETIC NERVE STIMULATION ON VOLUME, VASCULAR RESISTANCE, AND NOREPINEPHRINE OUTPUT IN THE ISOLATED PERFUSED SPLEEN OF THE CAT, AND ITS MODIFICATION BY COCAINE. J Pharmacol Exp Ther. 1964 Jan;143:57–63. [PubMed] [Google Scholar]
- TRENDELENBURG U. SUPERSENSITIVITY BY COCAINE TO DEXTROROTATORY ISOMERS OF NOREPINEPHRINE AND EPINEPHRINE. J Pharmacol Exp Ther. 1965 Jun;148:329–338. [PubMed] [Google Scholar]
- VANZWIETEN P. A., WIDHALM S., HERTTING G. INFLUENCE OF COCAINE AND OF PRETREATMENT WITH RESERPINE ON THE PRESSOR EFFECT AND THE TISSUE UPTAKE OF INJECTED DL-CATECHOLAMINES-2-H3. J Pharmacol Exp Ther. 1965 Jul;149:50–56. [PubMed] [Google Scholar]
- WHITBY L. G., AXELROD J., WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961 May;132:193–201. [PubMed] [Google Scholar]
- WHITBY L. G., HERTTING G., AXELROD J. Effect of cocaine on the disposition of noradrenaline labelled with tritium. Nature. 1960 Aug 13;187:604–605. doi: 10.1038/187604a0. [DOI] [PubMed] [Google Scholar]


