Abstract
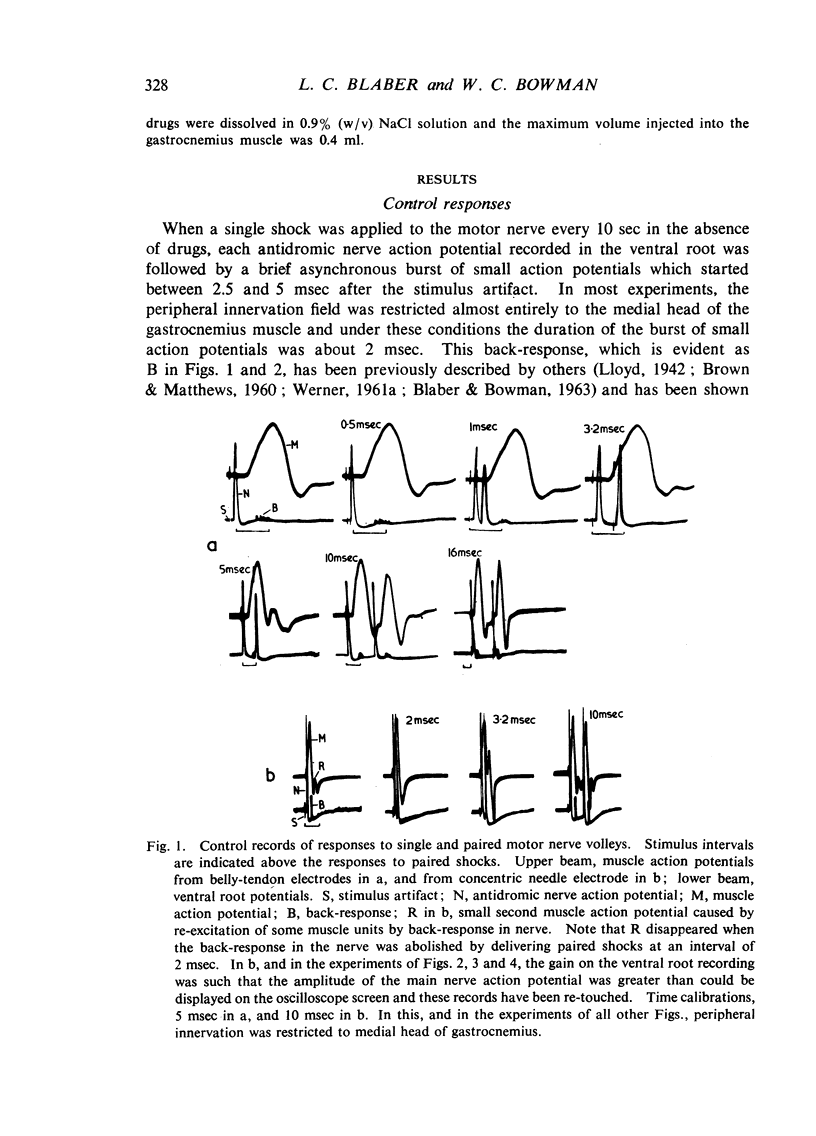
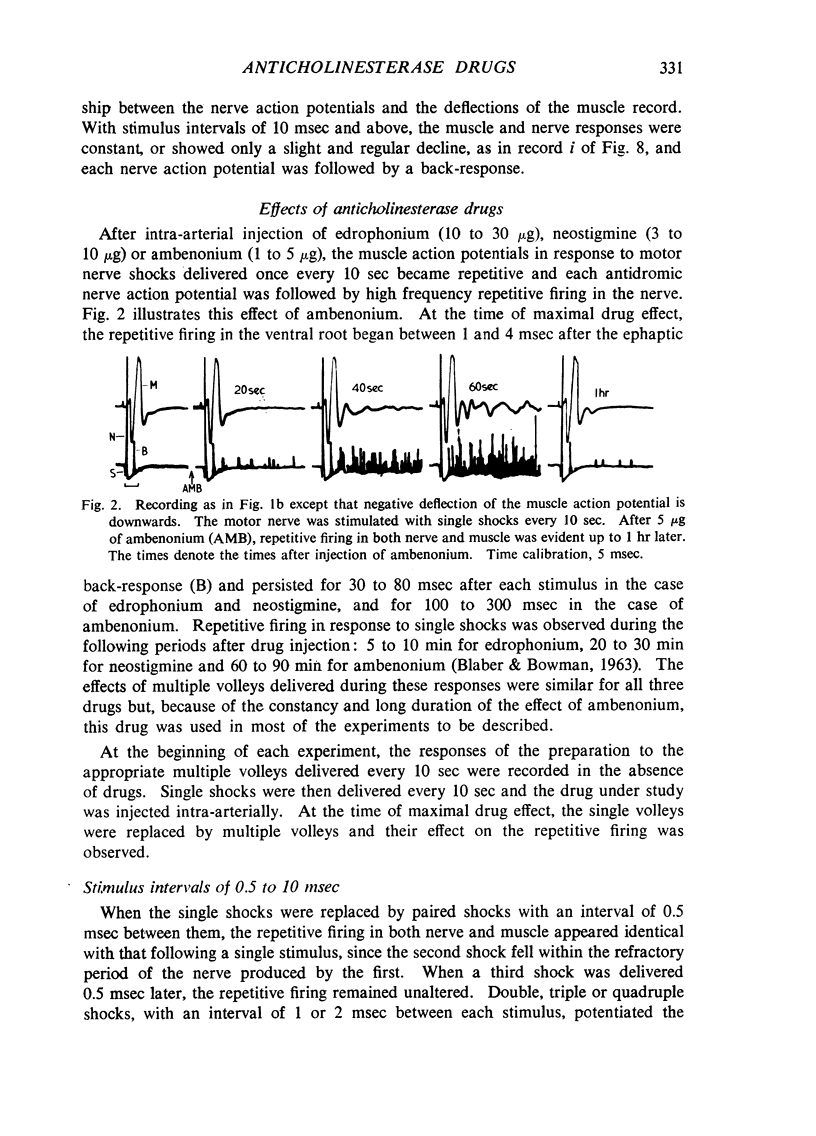
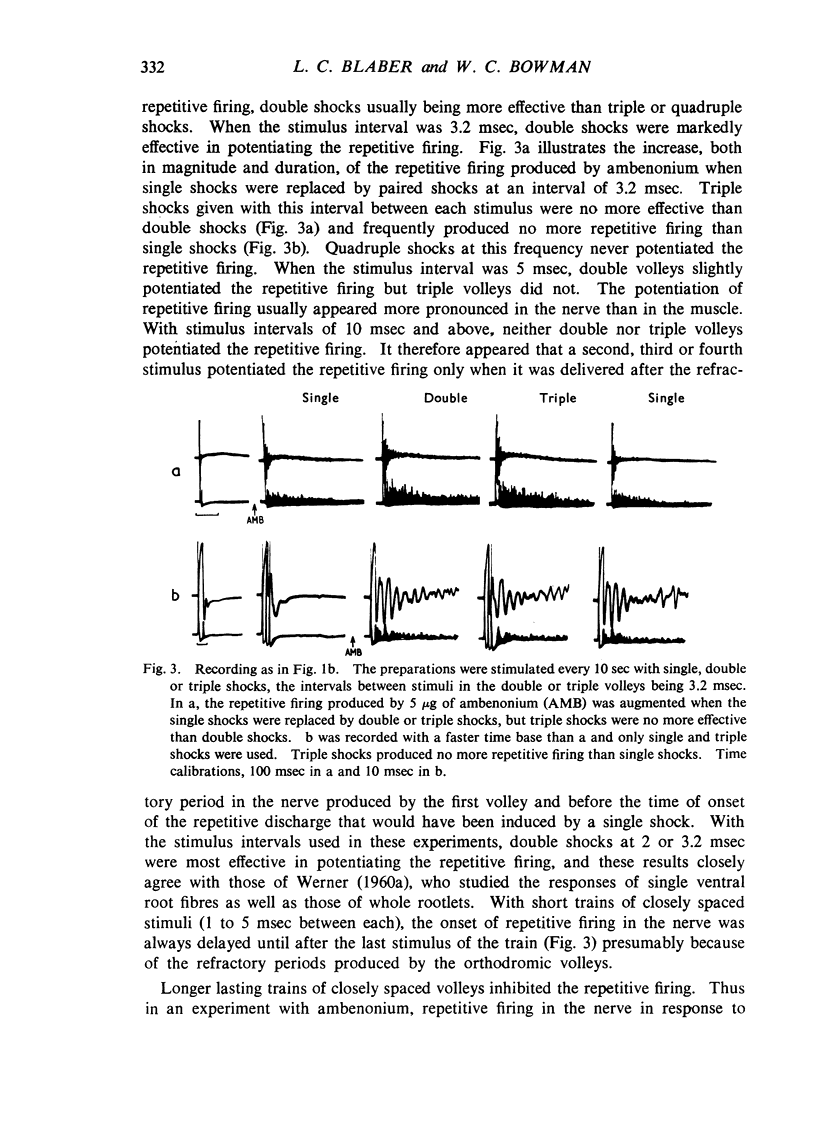
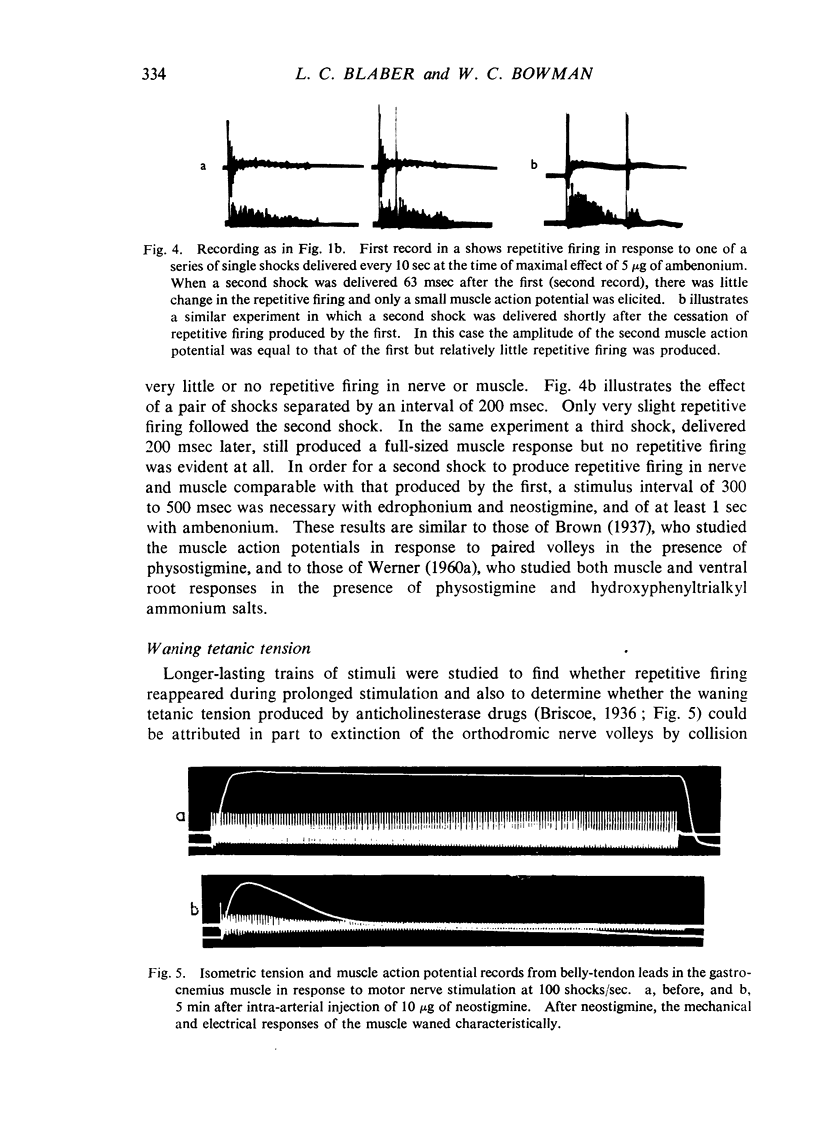
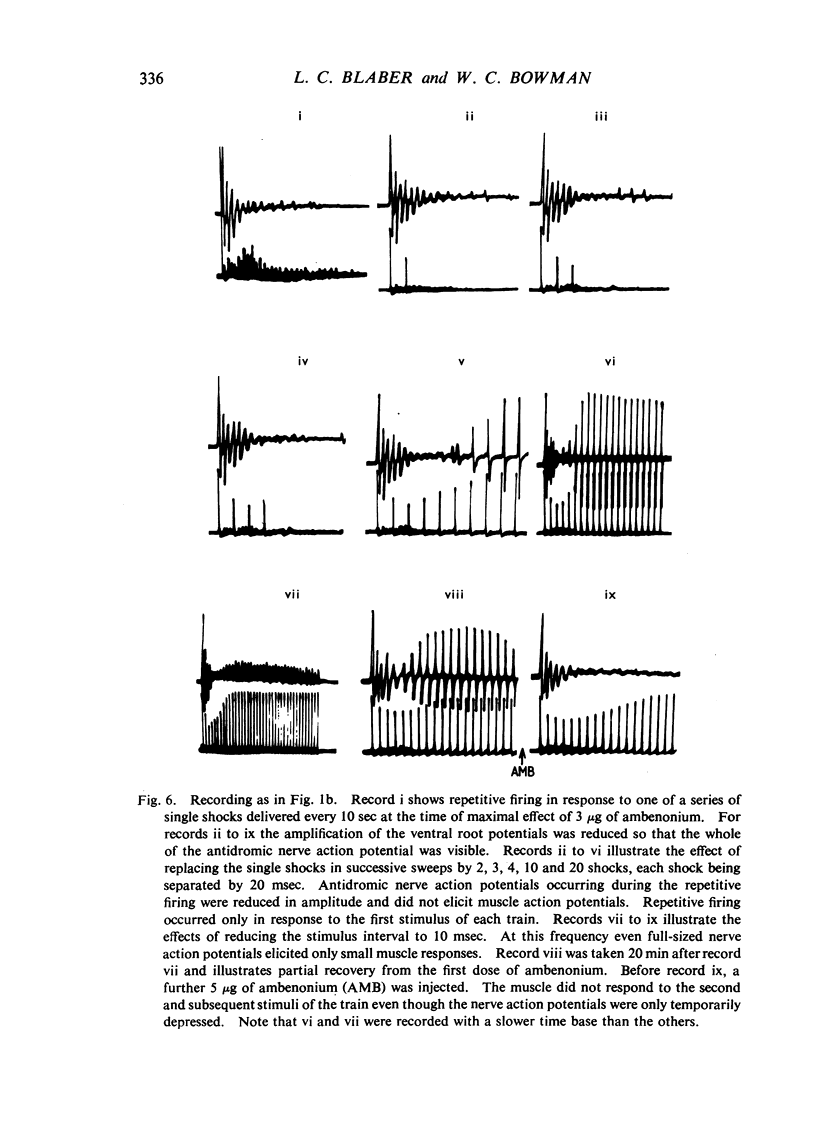
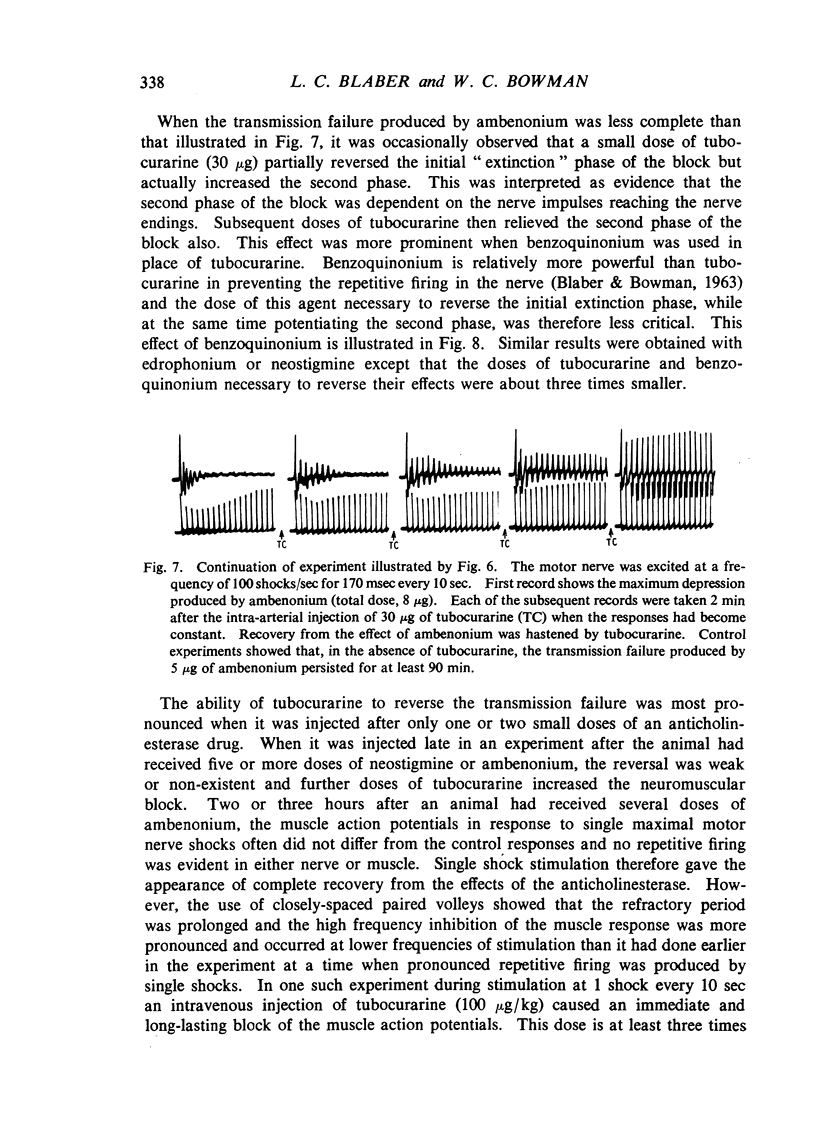
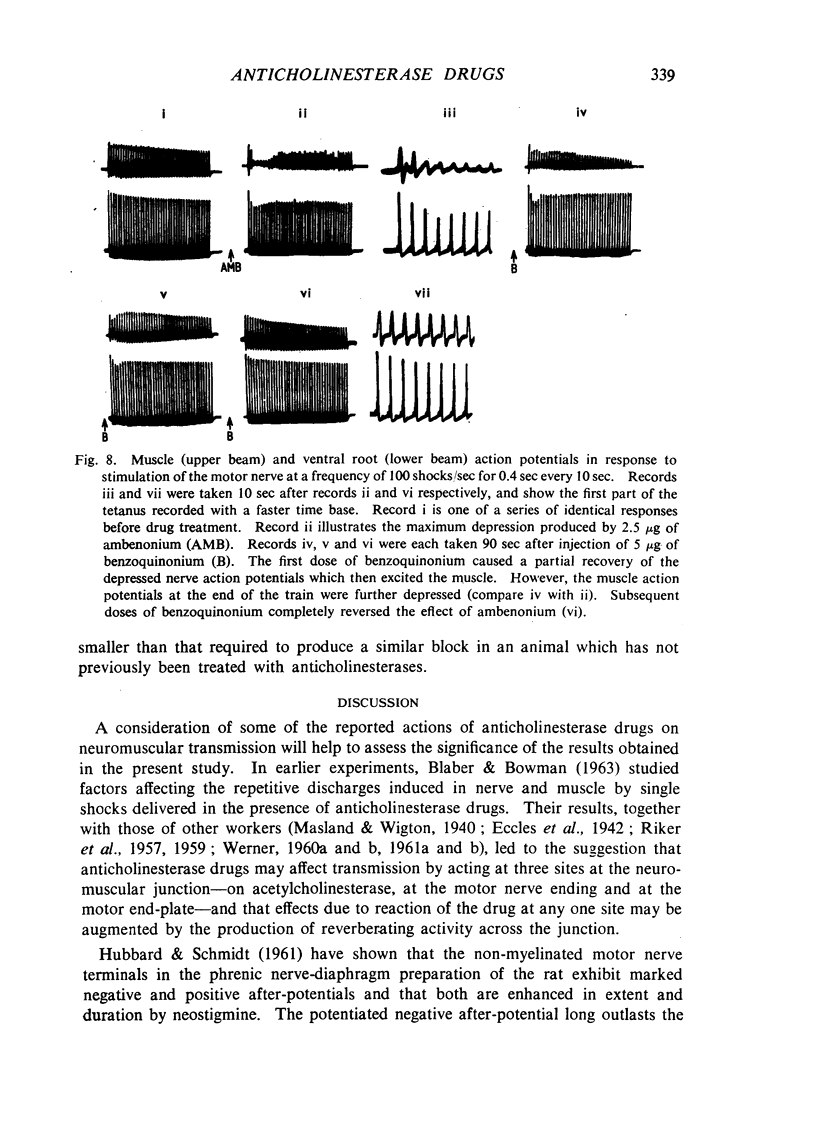
Repetitive discharges recorded from the ventral root and from the gastrocnemius muscle in response to single motor nerve shocks applied close to the muscle after injection of edrophonium, neostigmine or ambenonium were studied in cats anaesthetized with chloralose. Two closely spaced volleys with an interval of 1 to 5 msec between them produced more repetitive firing than did a single shock. With longer intervals, the repetitive firing was not potentiated by the second volley. All frequencies of tetanic stimulation depressed the repetitive firing and, for successive stimuli to produce a degree of repetitive firing equivalent to the first, it was necessary to stimulate at frequencies below 2 shocks/sec. With stimulation frequencies higher than 100 shocks/sec, repetitive firing did not occur unless the duration of the tetanus was shorter than about 30 msec when slight repetition followed the last stimulus of the train. With stimulation frequencies of 100 down to 20 shocks/sec, repetitive firing was produced only by the first volley of the tetanus. Subsequent nerve action potentials of the tetanus occurring during the repetitive firing in the nerve following the first volley were partially extinguished by collision with the back discharge. This effect contributed to the waning tetanus, which is characteristic of treatment with an anticholinesterase, but the main depression of tetanic contractions appeared to be a consequence of depolarization block through accumulating acetylcholine. Tubocurarine and benzoquinonium reversed the initial “extinction” phase of the depressed tetani by abolishing the repetitive discharge in the nerve and in larger doses reversed the secondary depressant phase presumably by reducing the excessive end-plate depolarization. The results are discussed in relation to the hypothesis that anticholinesterases may effect transmission by acting at three sites at the neuromuscular junction—on acetylcholinesterase, at the motor nerve ending and at the motor end-plate—and that reaction at any one site may be augmented by the production of reverberating activity across the junction.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLABER L. C., BOWMAN W. C. A comparison between the effects of edrophonium and choline in the skeletal muscles of the cat. Br J Pharmacol Chemother. 1959 Dec;14:456–466. doi: 10.1111/j.1476-5381.1959.tb00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLABER L. C., BOWMAN W. C. A comparison between the responses of avian and mammalian muscles to substances with facilitate neuromuscular transmission. Arch Int Pharmacodyn Ther. 1962 Jul 1;138:185–208. [PubMed] [Google Scholar]
- BOWMAN W. C. The neuromuscular blocking action of benzoquinonium chloride in the cat and in the hen. Br J Pharmacol Chemother. 1958 Dec;13(4):521–530. doi: 10.1111/j.1476-5381.1958.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. The effect on a muscle twitch of the back-response of its motor nerve fibres. J Physiol. 1960 Feb;150:332–346. doi: 10.1113/jphysiol.1960.sp006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L. Action potentials of normal mammalian muscle. Effects of acetylcholine and eserine. J Physiol. 1937 Mar 5;89(2):220–237. doi: 10.1113/jphysiol.1937.sp003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Dale H. H., Feldberg W. Reactions of the normal mammalian muscle to acetylcholine and to eserine. J Physiol. 1936 Sep 8;87(4):394–424. doi: 10.1113/jphysiol.1936.sp003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Harvey A. M. Neuro-muscular conduction in the fowl. J Physiol. 1938 Aug 15;93(3):285–300. doi: 10.1113/jphysiol.1938.sp003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Harvey A. M. Neuro-muscular transmission in the extrinsic muscles of the eye. J Physiol. 1941 Mar 25;99(3):379–399. doi: 10.1113/jphysiol.1941.sp003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., PATON W. D. The mechanisms of motor end-plate depolarization due to a cholinesterase-inhibiting drug. J Physiol. 1954 May 28;124(2):325–344. doi: 10.1113/jphysiol.1954.sp005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol. 1960 Mar;150:501–514. doi: 10.1113/jphysiol.1960.sp006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. Stimulation of motor nerve terminals. Nature. 1961 Sep 9;191:1103–1104. doi: 10.1038/1911103a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. The release of acetylcholine in the isolated rat diaphragm. J Physiol. 1961 Feb;155:246–262. doi: 10.1113/jphysiol.1961.sp006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURTHA E. F., MCNAMARA B. P., EDBERG L. J., BERGNER A. D., WILLS J. H. Studies on the pharmacology of tetraethylpyrophosphate. J Pharmacol Exp Ther. 1955 Nov;115(3):291–299. [PubMed] [Google Scholar]
- RIKER W. F., Jr, ROBERTS J., STANDAERT F. G., FUJIMORI H. The motor nerve terminal as the primary focus for drug-in-duced facilitation of neuromuscular transmission. J Pharmacol Exp Ther. 1957 Nov;121(3):286–312. [PubMed] [Google Scholar]
- WERNER G. Antidromic activity in motor nerves and its relation to a generator event in nerve terminals. J Neurophysiol. 1961 Jul;24:401–413. doi: 10.1152/jn.1961.24.4.401. [DOI] [PubMed] [Google Scholar]
- WERNER G. Generation of antidromic activity in motor nerves. J Neurophysiol. 1960 Jul;23:453–461. doi: 10.1152/jn.1960.23.4.453. [DOI] [PubMed] [Google Scholar]
- WERNER G. Neuromuscular facilitation and antidromic discharges in motor nerves: their relation to activity in motor nerve terminals. J Neurophysiol. 1960 Mar;23:171–187. doi: 10.1152/jn.1960.23.2.171. [DOI] [PubMed] [Google Scholar]
- WERNER G. Spontaneous miniature activity and gradation of transmission at the neuromuscular junction. Experientia. 1961 Feb 15;17:95–96. doi: 10.1007/BF02171449. [DOI] [PubMed] [Google Scholar]


