Abstract
1. Gas-liquid chromatographic methods were used to measure urinary acidic and alcoholic metabolites of L-DOPA, which had been administered in high oral dosage to patients with postencephalitic and idiopathic Parkinsonism.
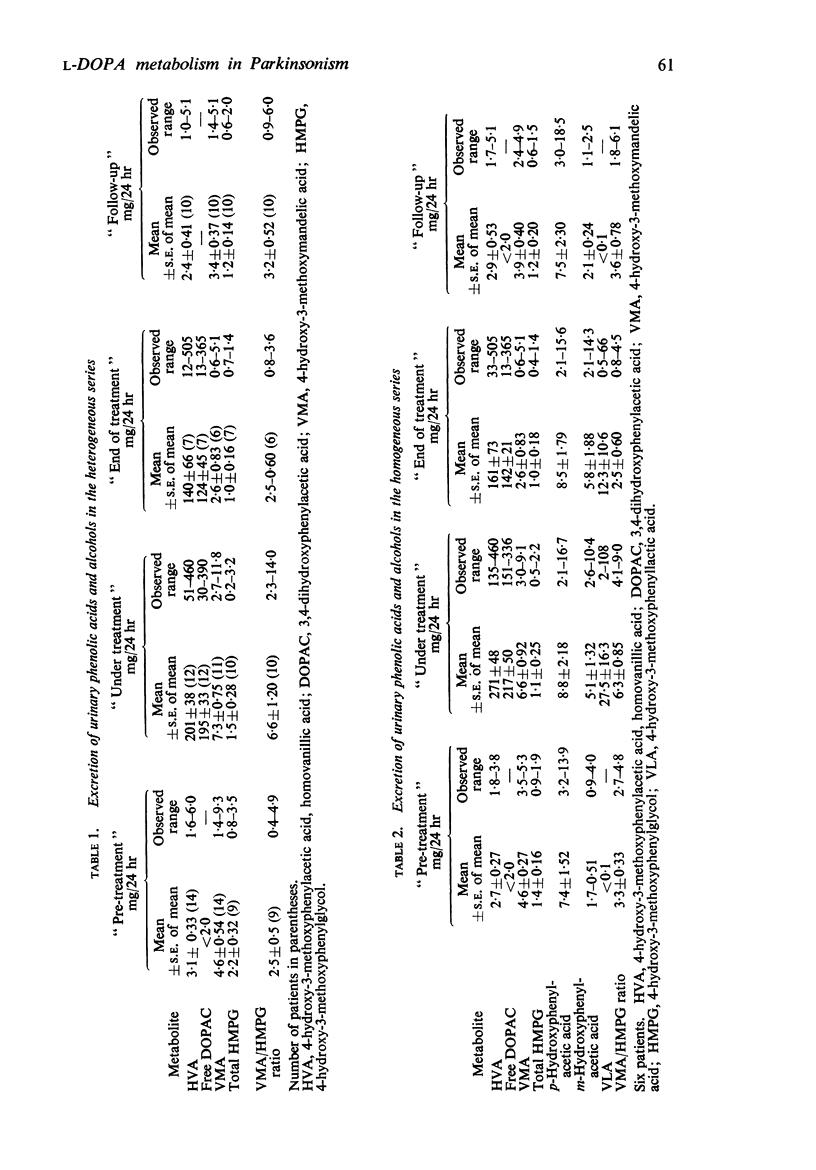
2. The output of these compounds was normal before treatment. During drug therapy, large quantities of the dopamine metabolites, homovanillic acid and dihydroxyphenylacetic acid, were excreted but traces only of 4-hydroxy-3-methoxyphenylethanol. Noradrenaline metabolites showed little change in output other than a small increase in 4-hydroxy-3-methoxymandelic acid.
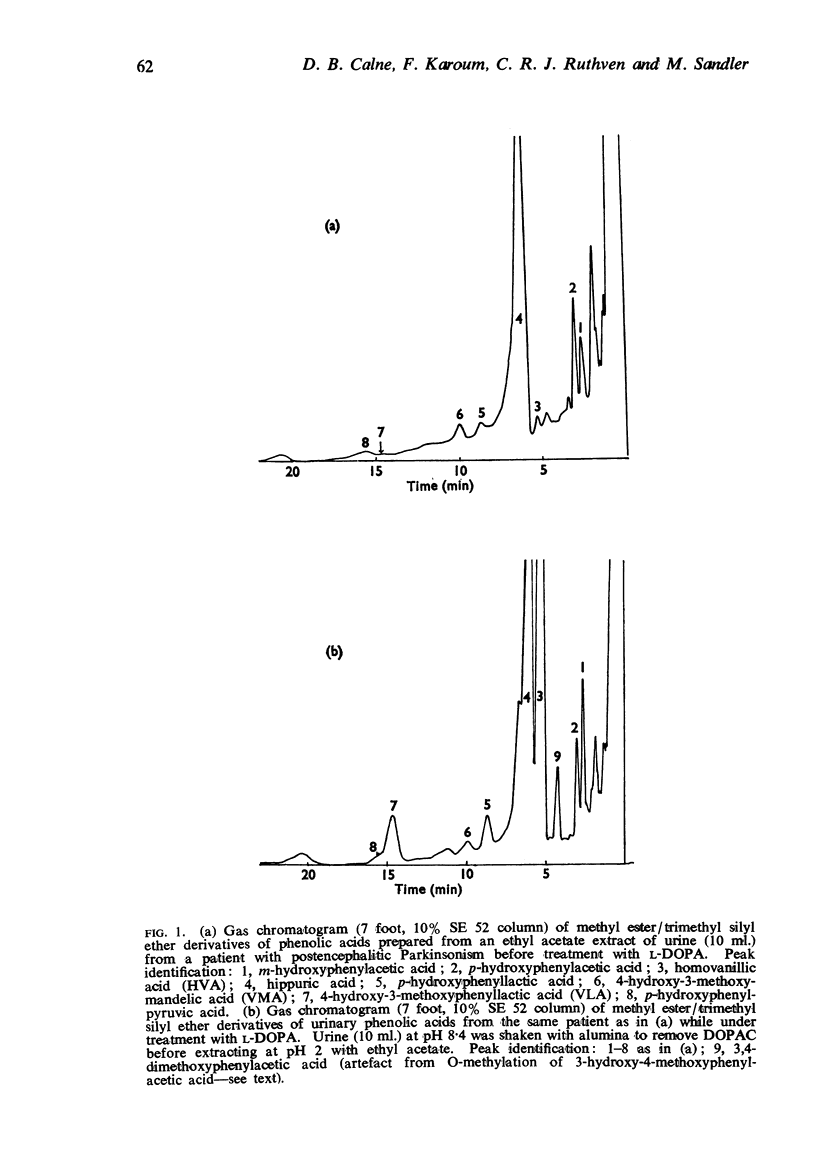
3. Information was obtained about a number of minor routes of degradation which might be implicated in the therapeutic action of L-DOPA. A raised output of m-hydroxyphenylacetic acid pointed to p-dehydroxylation of dihydroxyphenylacetic acid by gut flora. Evidence of transamination as a minor metabolic pathway was obtained by finding appreciable urinary levels of 4-hydroxy-3-methoxyphenyllactic acid. A keto-acid precursor of this compound may act as competitive inhibitor of an enzyme active in the normal degradation route of tyrosine, p-hydroxyphenylpyruvic acid oxidase, for increased amounts of p-hydroxyphenyllactic acid, the major metabolic derivative of p-hydroxyphenylpyruvic acid, accumulated in the urine during DOPA treatment.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBEAU A., MURPHY G. F., SOURKES T. L. Excretion of dopamine in diseases of basal ganglia. Science. 1961 May 26;133(3465):1706–1707. doi: 10.1126/science.133.3465.1706-a. [DOI] [PubMed] [Google Scholar]
- BOOTH A. N., EMERSON O. H., JONES F. T., DEEDS F. Urinary metabolites of caffeic and chlorogenic acids. J Biol Chem. 1957 Nov;229(1):51–59. [PubMed] [Google Scholar]
- Breese G. R., Chase T. N., Kopin I. J. Metabolism of some phenylethylamines and their beta-hydroxylated analogs in brain. J Pharmacol Exp Ther. 1969 Jan;165(1):9–13. [PubMed] [Google Scholar]
- CAMMARATA P. S., COHEN P. P. The scope of the transamination reaction in animal tissues. J Biol Chem. 1950 Nov;187(1):439–452. [PubMed] [Google Scholar]
- Calne D. B., Stern G. M., Laurence D. R., Sharkey J., Armitage P. L-dopa in postencephalitic parkinsonism. Lancet. 1969 Apr 12;1(7598):744–746. doi: 10.1016/s0140-6736(69)91751-6. [DOI] [PubMed] [Google Scholar]
- Cotzias G. C., Papavasiliou P. S., Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969 Feb 13;280(7):337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- DEEDS F., BOOTH A. N., JONES F. T. Methylation and dehydroxylation of phenolic compounds by rats and rabbits. J Biol Chem. 1957 Apr;225(2):615–621. [PubMed] [Google Scholar]
- Dalgliesh C. E., Horning E. C., Horning M. G., Knox K. L., Yarger K. A gas-liquid-chromatographic procedure for separating a wide range of metabolites occuring in urine or tissue extracts. Biochem J. 1966 Dec;101(3):792–810. doi: 10.1042/bj1010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONNUM F., HAAVALDSEN R., TANGEN O. TRANSAMINATION OF AROMATIC AMINO ACIDS IN RAT BRAIN. J Neurochem. 1964 Feb;11:109–118. doi: 10.1111/j.1471-4159.1964.tb06747.x. [DOI] [PubMed] [Google Scholar]
- GJESSING L. R. STUDIES OF FUNCTIONAL NEURAL TUMORS. V. URINARY EXCRETION OF 3-METHOXY-4HYDROXYPHENYL-LACTIC ACID. Scand J Clin Lab Invest. 1963;15:649–653. doi: 10.3109/00365516309051348. [DOI] [PubMed] [Google Scholar]
- Gey K. F., Pletscher A. Distribution and metabolism of DL-3,4-dihydroxy[2-14C]-phenylalanine in rat tissues. Biochem J. 1964 Aug;92(2):300–308. doi: 10.1042/bj0920300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967 Sep 29;157(3796):1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Karoum F., Anah C. O., Ruthven C. R., Sandler M. Further observations on the gas-chromatographic measurement of urinary phenolic and indolic metabolites. Clin Chim Acta. 1969 Jun;24(3):341–348. doi: 10.1016/0009-8981(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Karoum F., Ruthven C. R., Sandler M. Gas chromatographic measurement of phenolic acids and alcohols in human urine. Clin Chim Acta. 1968;20(3):427–437. doi: 10.1016/0009-8981(68)90300-8. [DOI] [PubMed] [Google Scholar]
- MCGEER P. L., BOULDING J. E., GIBSON W. C., FOULKES R. G. Drug-induced extrapyramidal reactions. Treatment with diphenhydramine hydrochloride and dihydroxyphenylalanine. JAMA. 1961 Sep 9;177:665–670. doi: 10.1001/jama.1961.03040360001001. [DOI] [PubMed] [Google Scholar]
- Pletscher A., Bartholini G., Tissot R. Metabolic fate of l-[14C] DOPA in cerebrospinal fluid and blood plasma of humans. Brain Res. 1967 Feb;4(1):106–109. doi: 10.1016/0006-8993(67)90154-0. [DOI] [PubMed] [Google Scholar]
- SHAW K. N., MCMILLAN A., ARMSTRONG M. D. The metabolism of 3, 4-dihydroxyphenylalanine. J Biol Chem. 1957 May;226(1):255–266. [PubMed] [Google Scholar]
- SOURKES T. L., DENTON R. L., MURPHY G. F., CHAVEZ B., SAINT CYR S. The excretion of dihydroxyphenylalanine, dopamine, and dihydroxyphenylacetic acid in neuroblastoma. Pediatrics. 1963 Apr;31:660–668. [PubMed] [Google Scholar]
- SOURKES T. L., PIVNICKI D., BROWN W. T., WISEMAN-DISTLER H., MURPHY G. F., SANKOFF I., SAINTCYR S. A CLINICAL AND METABOLIC STUDY OF DOPA (3, 4-DIHYDROXYPHENYLALANINE) AND METHYLDOPA IN HUNTINGTON'S CHOREA. Psychiatr Neurol (Basel) 1965;149:7–27. doi: 10.1159/000128799. [DOI] [PubMed] [Google Scholar]
- Sandler M., Ruthven C. R. The biosynthesis and metabolism of the catecholamines. Prog Med Chem. 1969;6:200–265. doi: 10.1016/s0079-6468(08)70199-1. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Drug metabolism by intestinal microorganisms. J Pharm Sci. 1968 Dec;57(12):2021–2037. doi: 10.1002/jps.2600571202. [DOI] [PubMed] [Google Scholar]
- Shulgin A. T., Sargent T., Naranjo C. Structure--activity relationships of one-ring psychotomimetics. Nature. 1969 Feb 8;221(5180):537–541. doi: 10.1038/221537a0. [DOI] [PubMed] [Google Scholar]
- Smith P. Metabolism of dihydroxyphenylalanine in human subjects. Nature. 1967 Feb 25;213(5078):802–803. doi: 10.1038/213802a0. [DOI] [PubMed] [Google Scholar]
- Weber W. W., Zannoni V. G. Reduction of phenylpyruvic acids to phenyllactic acids in mammalian tissues. J Biol Chem. 1966 Mar 25;241(6):1345–1349. [PubMed] [Google Scholar]
- Zannoni V. G., Weber W. W. Isolation and properties of aromatic alpha-keto acid reductase. J Biol Chem. 1966 Mar 25;241(6):1340–1344. [PubMed] [Google Scholar]
- von STUDNITZ [Methodical and clinical studies on the excretion of 3-methoxy-4-hydroxymendelic acid in the urine]. Scand J Clin Lab Invest. 1960;12 (Suppl 48):3–73. [PubMed] [Google Scholar]
- von Studnitz W. Zur Frage des Vorkommens von 3-Hydroxy-4-methoxymandelsäure beim normalen und gesteigerten Katecholaminstoffwechsel. Klin Wochenschr. 1967 Mar 15;45(6):307–308. doi: 10.1007/BF01747101. [DOI] [PubMed] [Google Scholar]


