Abstract
Lignin biodegradation in a variety of natural materials was examined using specifically labeled synthetic 14C-lignins. Natural materials included soils, sediments, silage, steer bedding, and rumen contents. Both aerobic and anaerobic incubations were used. No 14C-labeled lignin biodegradation to labeled gaseous products under anaerobic conditions was observed. Aerobic 14C-labeled lignin mineralization varied with respect to type of natural material used, site, soil type and horizon, and temperature. The greatest observed degradation occurred in a soil from Yellowstone National Park and amounted to over 42% conversion of total radioactivity to 14CO2 during 78 days of incubation. Amounts of 14C-labeled lignin mineralization in Wisconsin soils and sediments were significantly correlated with organic carbon, organic nitrogen, nitrate nitrogen, exchangeable calcium, and exchangeable potassium.
Full text
PDF

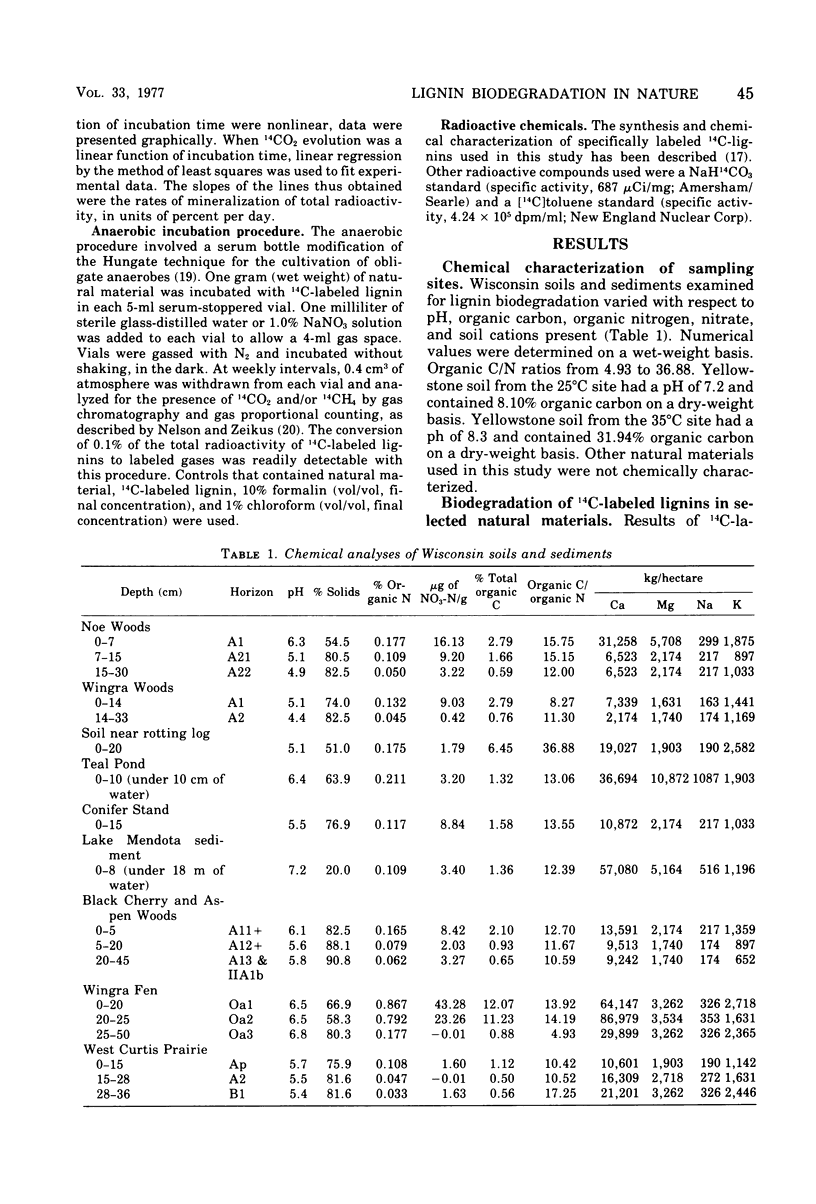
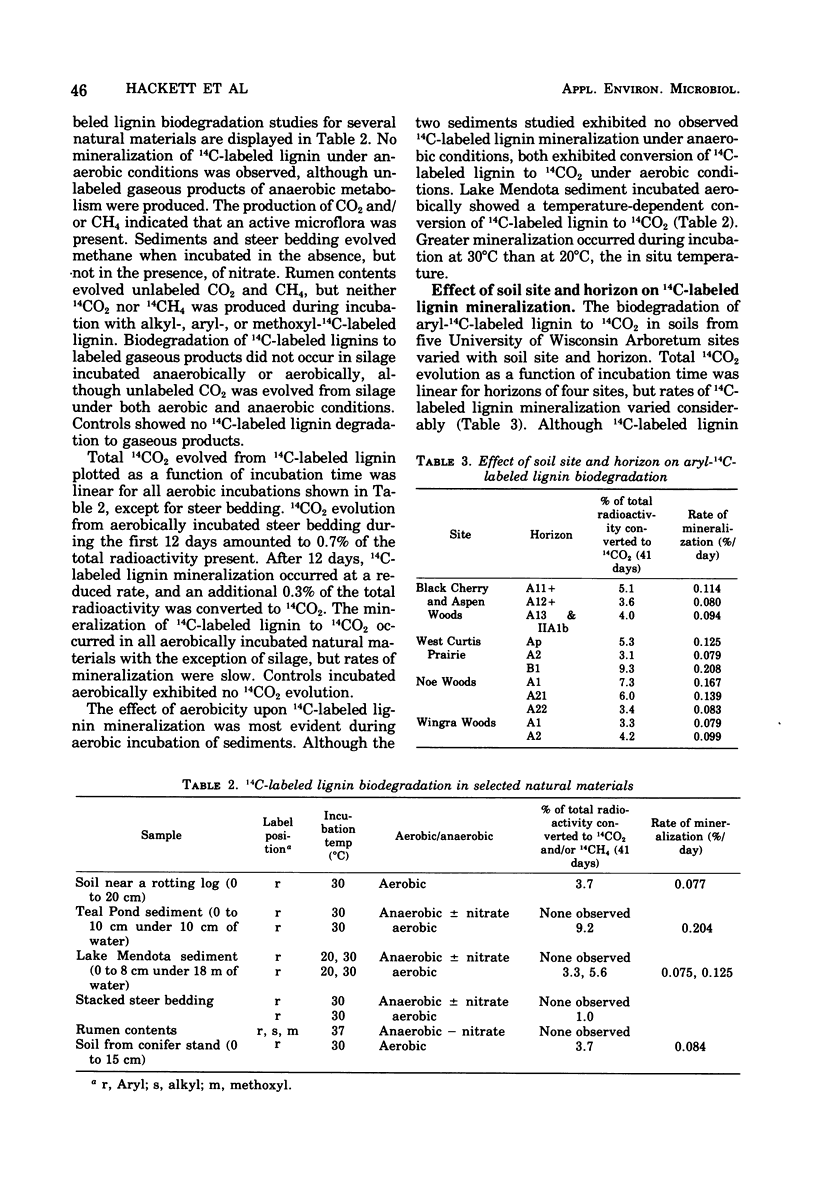
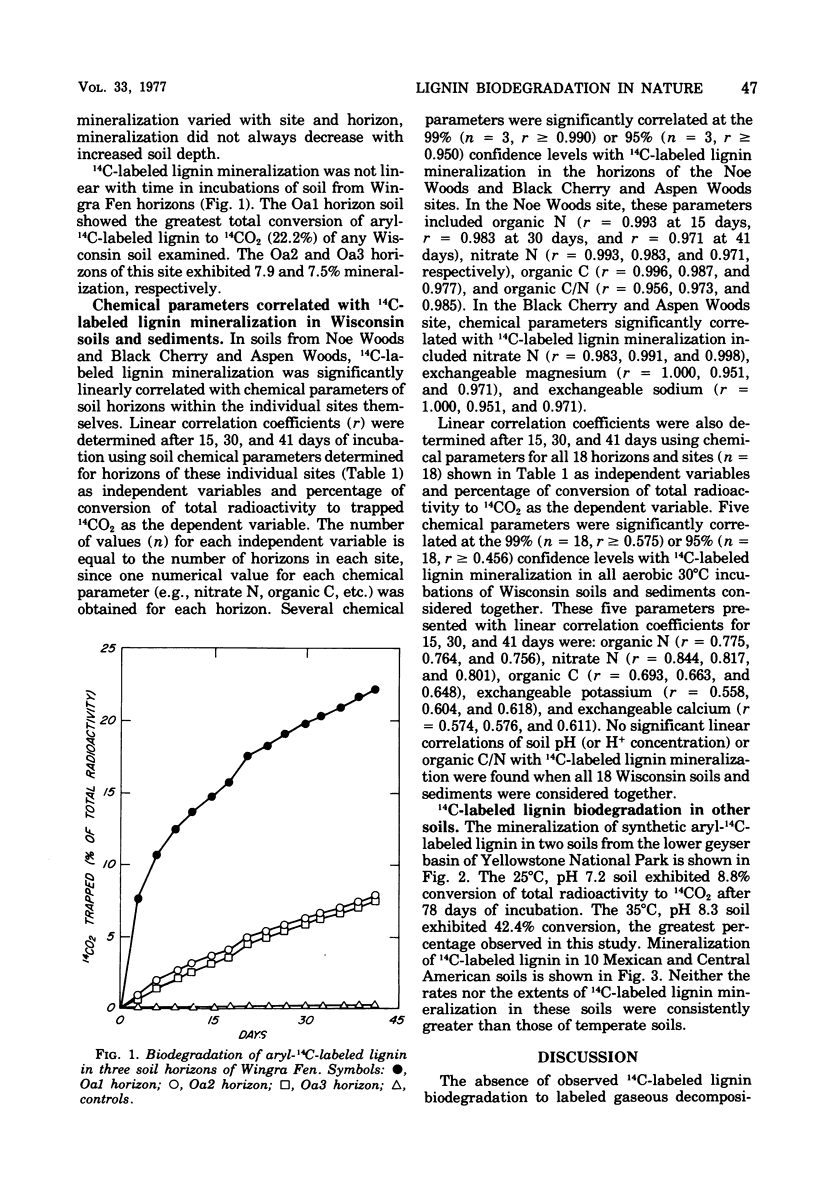
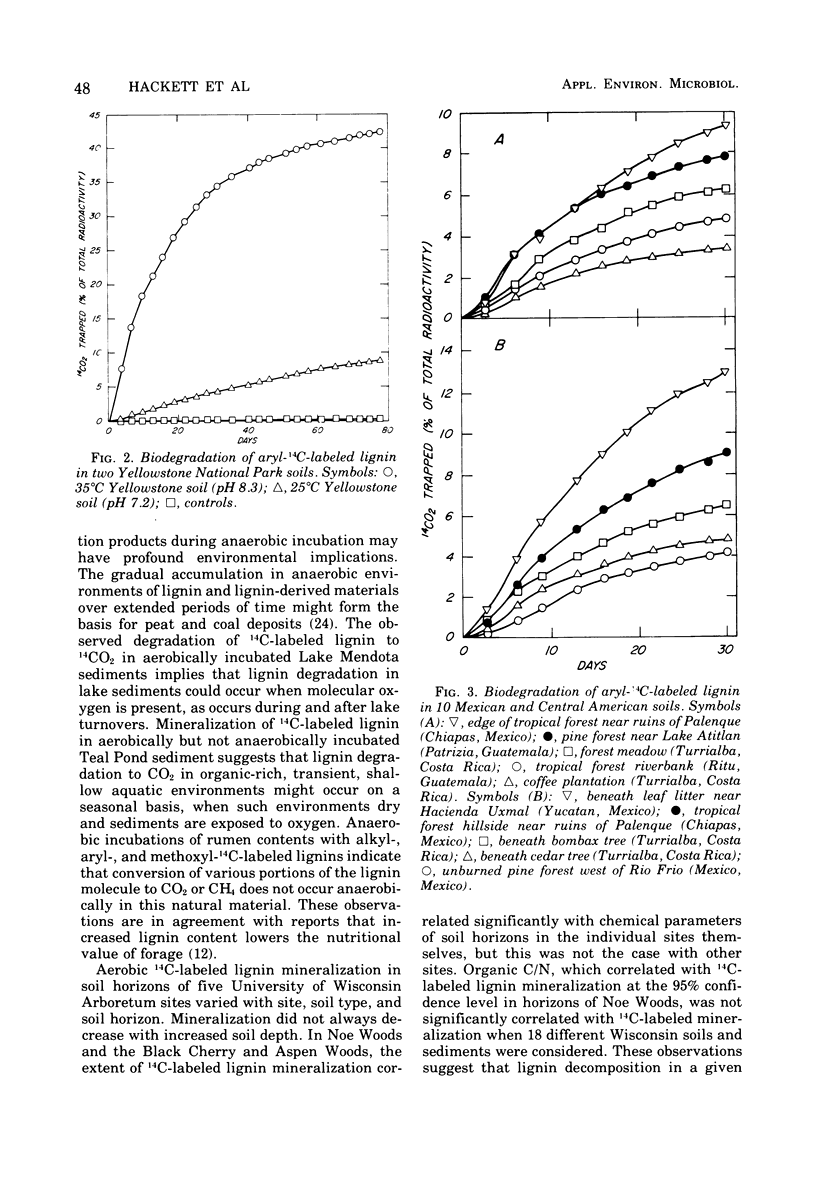



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Zeikus J. G. Requirement for a growth substrate during lignin decomposition by two wood-rotting fungi. Appl Environ Microbiol. 1976 Jul;32(1):192–194. doi: 10.1128/aem.32.1.192-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


