Abstract
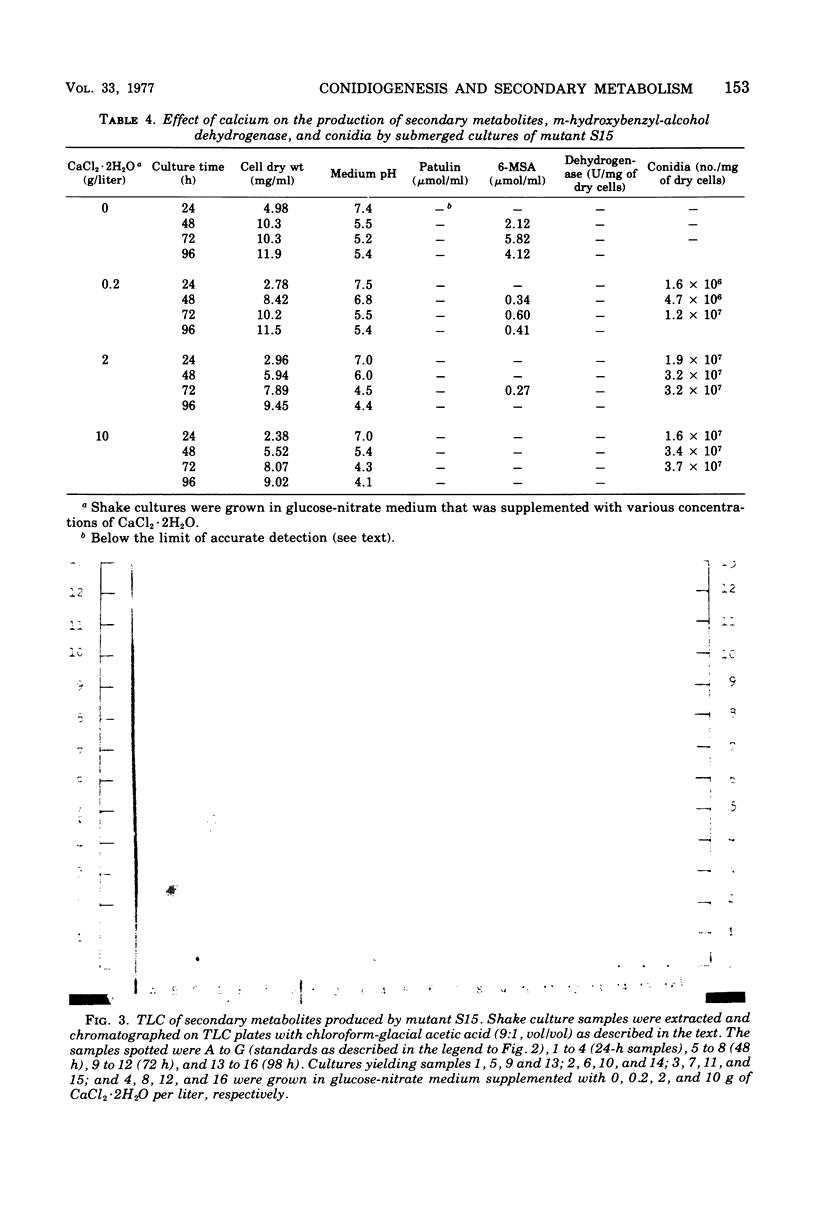
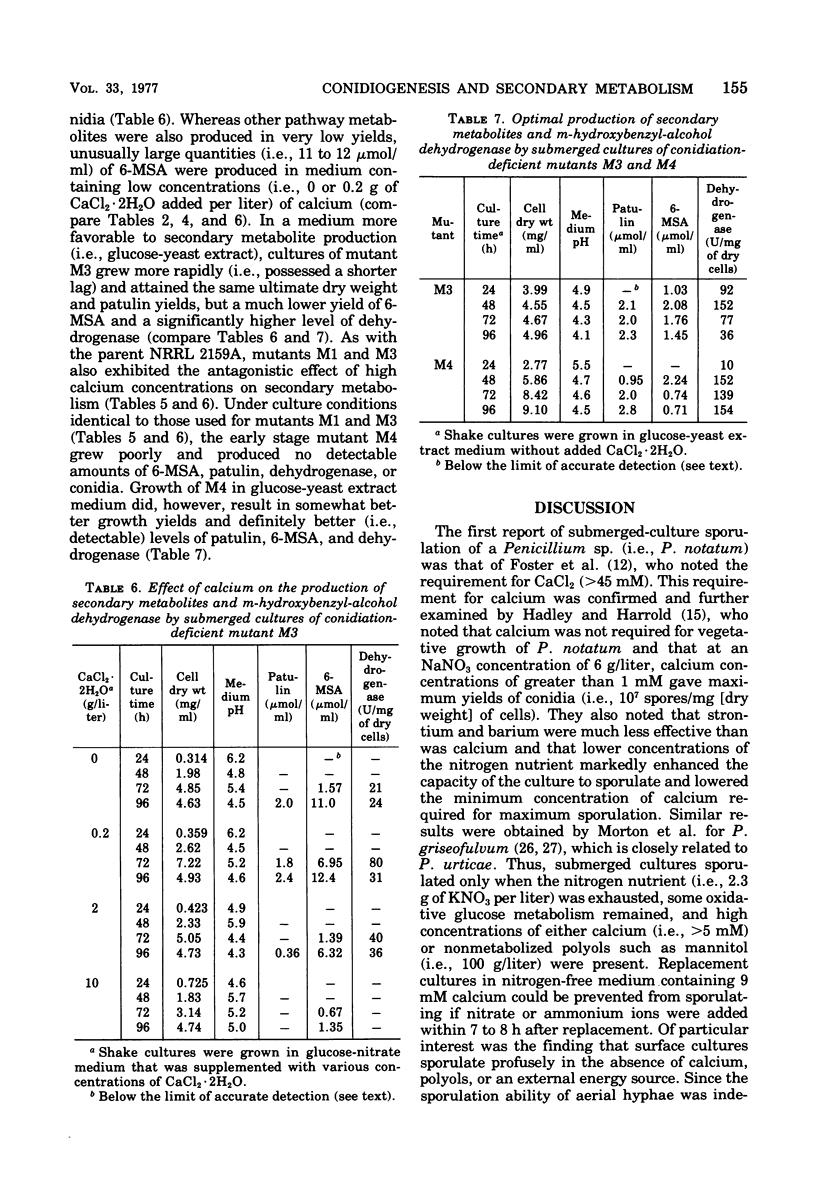
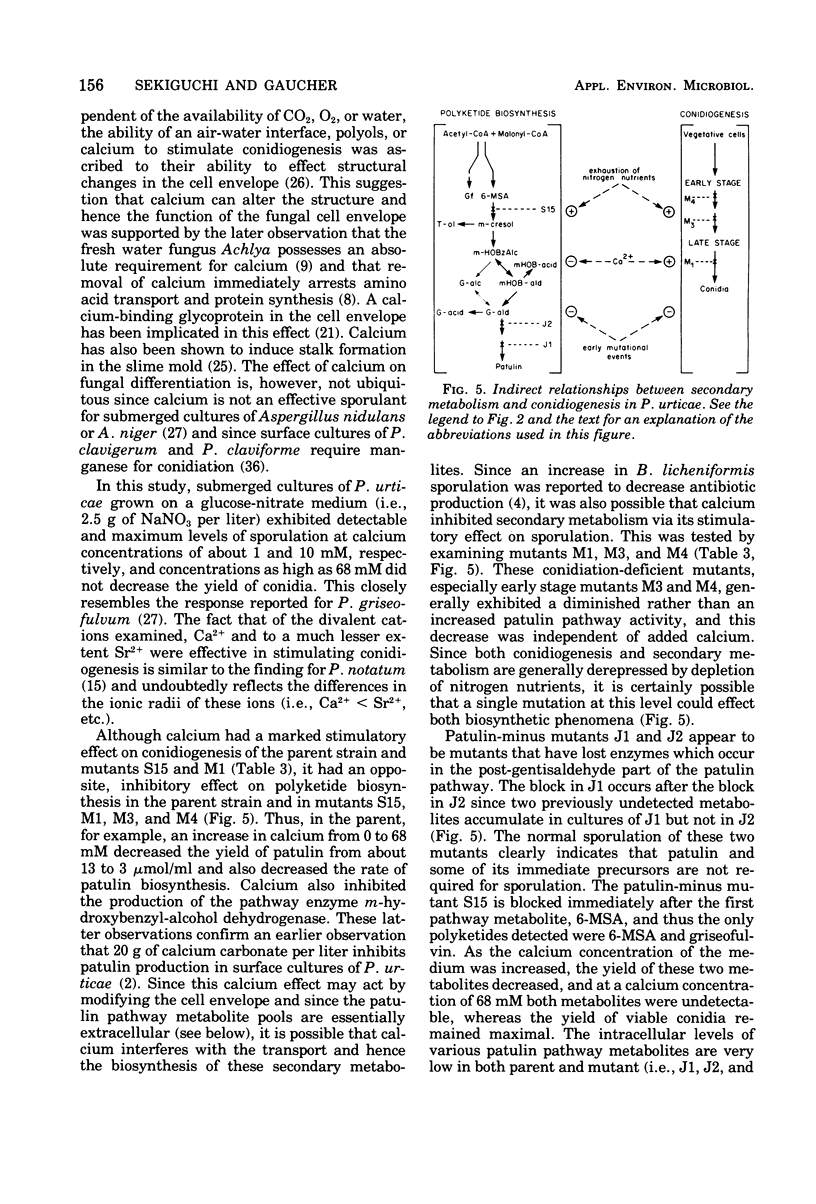
Submerged cultures of Penicillium urticae (NRRL 2159A) produced the antibiotics patulin and griseofulvin when grown in a glucose-nitrate medium. A high concentration of calcium (i.e., 68 mM) inhibited the production of both antibiotics while stimulating conidiogenesis. Conidial mutants that were defective in an early stage of conidiogenesis produced markedly less patulin, even under growth conditions that favored secondary metabolism. A mutant which lacked the ability to produce the patulin pathway metabolites m-cresol, toluquinol, m-hydroxybenzyl-alcohol, m-hydroxybenzaldehyde, gentisaldehyde, gentisyl alcohol, gentisic acid and patulin, as well as the pathway enzyme m-hydroxybenzyl-alcohol dehydrogenase, still produced yields of conidia that were equivalent to or greater than those of the parent strain. Other mutants which were blocked at later steps of the patulin pathway also produced conidia. These results indicate that patulin and the other related secondary metabolites noted above are not a prerequisite to conidiogenesis in P. urticae. Environmental and developmental factors such as calcium levels and conidiogenesis do, however, indirectly affect the production of patulin pathway metabolites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biochemical ecology of microorganisms. Annu Rev Microbiol. 1971;25:361–392. doi: 10.1146/annurev.mi.25.100171.002045. [DOI] [PubMed] [Google Scholar]
- BASSETT E. W., TANENBAUM S. W. The biosynthesis of patulin. II. The general physiology of several strains of Penicillium patulum. Biochim Biophys Acta. 1958 May;28(2):247–260. doi: 10.1016/0006-3002(58)90470-0. [DOI] [PubMed] [Google Scholar]
- Bent K. J. Electrophoresis of proteins of 3 Penicillium species on acrylamide gels. J Gen Microbiol. 1967 Nov;49(2):195–200. doi: 10.1099/00221287-49-2-195. [DOI] [PubMed] [Google Scholar]
- Cameron L. E., LéJohn H. B. Ca 2+ is a specific regulator of amino acid transport and protein synthesis in the water-mould achyla. Biochem Biophys Res Commun. 1972 Jul 11;48(1):181–189. doi: 10.1016/0006-291x(72)90360-9. [DOI] [PubMed] [Google Scholar]
- Cameron L. E., LéJohn H. B. On the involvement of calcium in amino acid transport and growth of the fungus Achlya. J Biol Chem. 1972 Aug 10;247(15):4729–4739. [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. Conversion of 6-methylsalicylic acid into patulin by Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1102–1107. doi: 10.1021/bi00756a025. [DOI] [PubMed] [Google Scholar]
- Foster J. W., McDaniel L. E., Woodruff H. B., Stokes J. L. Microbiological Aspects of Penicillin: V. Conidiospore Formation in Submerged Cultures of Penicillium Notatum. J Bacteriol. 1945 Sep;50(3):365–368. doi: 10.1128/jb.50.3.365-368.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher G. M. m-Hydroxybenzyl-alcohol dehydrogenase. Methods Enzymol. 1975;43:540–548. doi: 10.1016/0076-6879(75)43116-0. [DOI] [PubMed] [Google Scholar]
- Gooday G. W. Fungal sex hormones. Annu Rev Biochem. 1974;43(0):35–87. doi: 10.1146/annurev.bi.43.070174.000343. [DOI] [PubMed] [Google Scholar]
- Ichikawa T., Date M., Ishikura T., Ozaki A. Improvement of kasugamycin-producing strain by the agar piece method and the prototroph method. Folia Microbiol (Praha) 1971;16(3):218–224. doi: 10.1007/BF02884210. [DOI] [PubMed] [Google Scholar]
- Jicínská E. Note on study of the sporulation of fungi: endotrophic sporulation in the genus Penicillium. Folia Microbiol (Praha) 1968;13(5):401–409. doi: 10.1007/BF02869190. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Littau V., Lipmann F. The relation between sporulation and the induction of antibiotic synthesis and of amino acid uptake in Bacillus brevis. J Cell Biol. 1975 Aug;66(2):233–242. doi: 10.1083/jcb.66.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light R. J., Vogel G. 6-Methylsalicylic acid (2,6-cresotic acid) decarboxylase. Methods Enzymol. 1975;43:530–540. doi: 10.1016/0076-6879(75)43115-9. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B., Cameron L. E., Stevenson R. M., Meuser R. U. Influence of cytokinins and sulfhydryl group-reacting agents on calcium transport in fungi. J Biol Chem. 1974 Jul 10;249(13):4016–4020. [PubMed] [Google Scholar]
- Maeda Y., Maeda M. The calcium content of the cellular slime mold, Dictyostelium discoideum, during development and differentiation. Exp Cell Res. 1973 Nov;82(1):125–130. doi: 10.1016/0014-4827(73)90253-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Vogel G., Krippahl G., Lynen F. Patulin biosynthesis: the role of mixed-function oxidases in the hydroxylation of m-cresol. Eur J Biochem. 1974 Nov 15;49(2):443–455. doi: 10.1111/j.1432-1033.1974.tb03849.x. [DOI] [PubMed] [Google Scholar]
- Ristow H., Schazschneider B., Bauer K., Kleikauf H. Tyrocidine and the linear gramicidin. Do these peptide antibiotics play an antagonistic regulative role in sporulation? Biochim Biophys Acta. 1975 May 1;390(2):246–252. [PubMed] [Google Scholar]
- Robinson S., Winnik H. Z. Motivation for the addiction to amphetamine and reducing drugs. Isr Ann Psychiatr Relat Discip. 1969 Nov;7(2):213–222. [PubMed] [Google Scholar]
- Sadoff H. L. The antibiotics of Bacillus species: their possible roles in sporulation. Prog Ind Microbiol. 1972;11:1–27. [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M., Costerton J. W. Microcycle conidiation in Penicillium urticae: an ultrastructural investigation of conidiogenesis. Can J Microbiol. 1975 Dec;21(12):2069–2083. doi: 10.1139/m75-296. [DOI] [PubMed] [Google Scholar]
- Tinnell W. H., Jefferson B. L., Benoit R. E. The organic nitrogen exigency of and effects of manganese on coremia production in Penicillium clavigerum and Penicillium claviforme. Can J Microbiol. 1974 Jan;20(1):91–96. doi: 10.1139/m74-014. [DOI] [PubMed] [Google Scholar]
- Vogel G., Lynen F. 6-Methylsalicylic acid synthetase. Methods Enzymol. 1975;43:520–530. doi: 10.1016/0076-6879(75)43114-7. [DOI] [PubMed] [Google Scholar]
- Wolf J. C., Mirocha C. J. Regulation of sexual reproduction in Gibberella zeae (Fusarium roxeum "graminearum") by F-2 (Zearalenone). Can J Microbiol. 1973 Jun;19(6):725–734. doi: 10.1139/m73-117. [DOI] [PubMed] [Google Scholar]
- Zimmer T. L., Laland S. G. Gramicidine S synthetase. Methods Enzymol. 1975;43:567–579. doi: 10.1016/0076-6879(75)43119-6. [DOI] [PubMed] [Google Scholar]