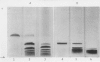
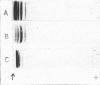
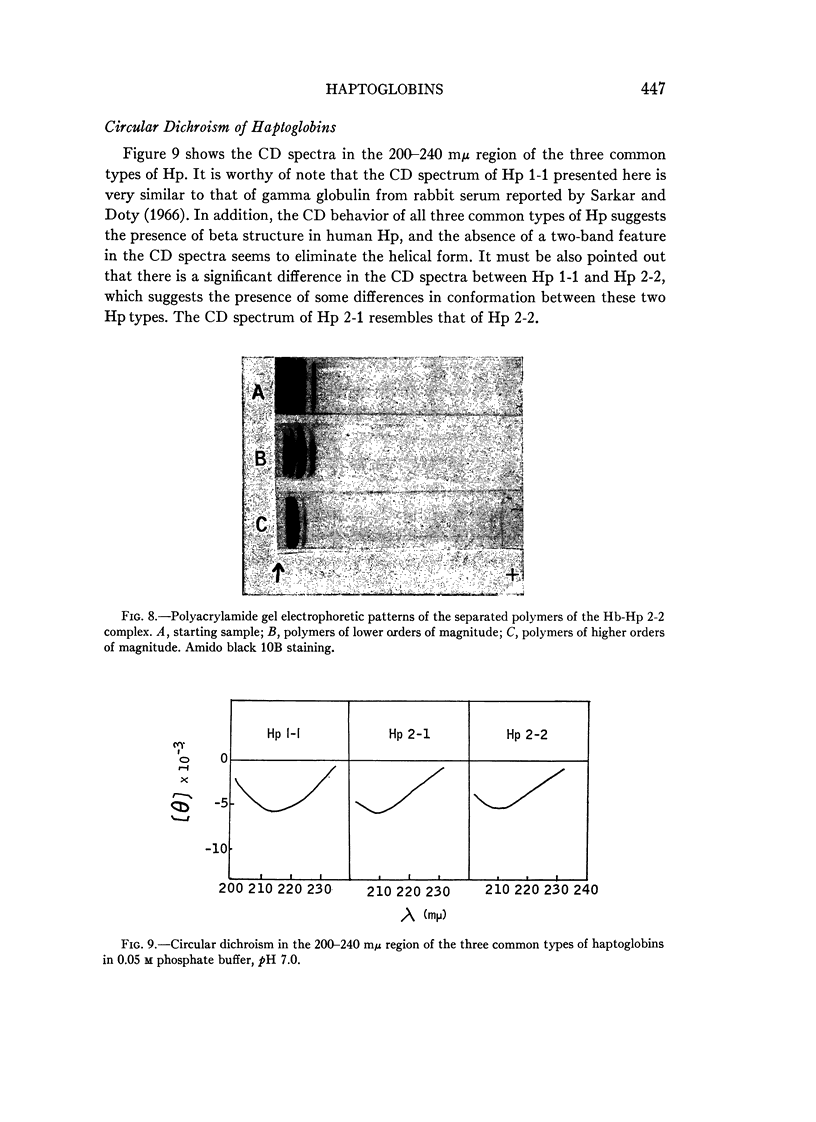
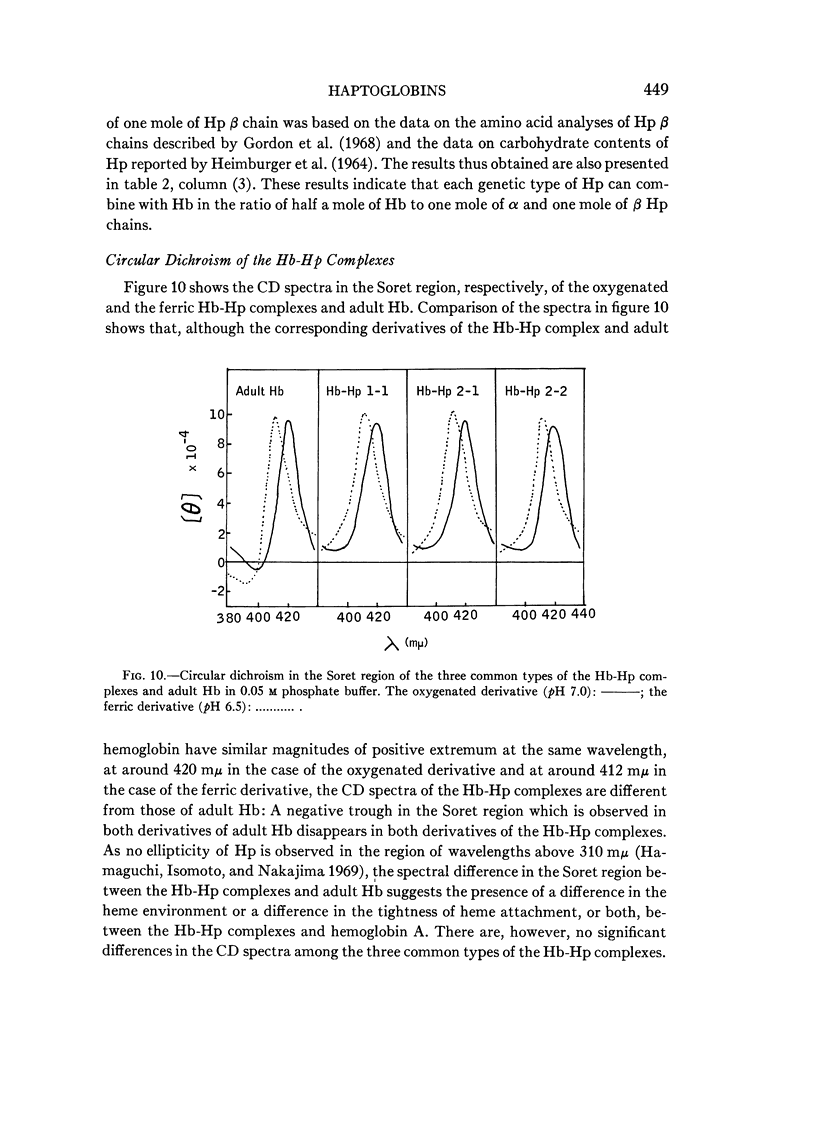
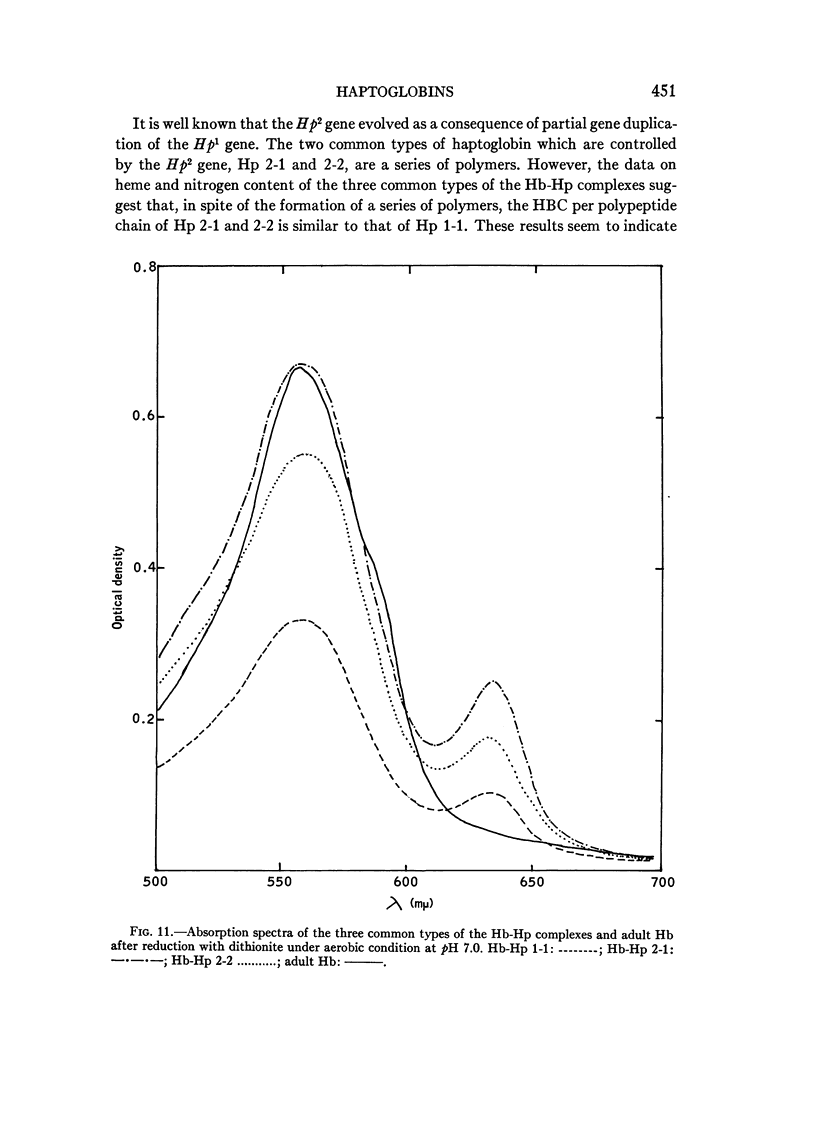
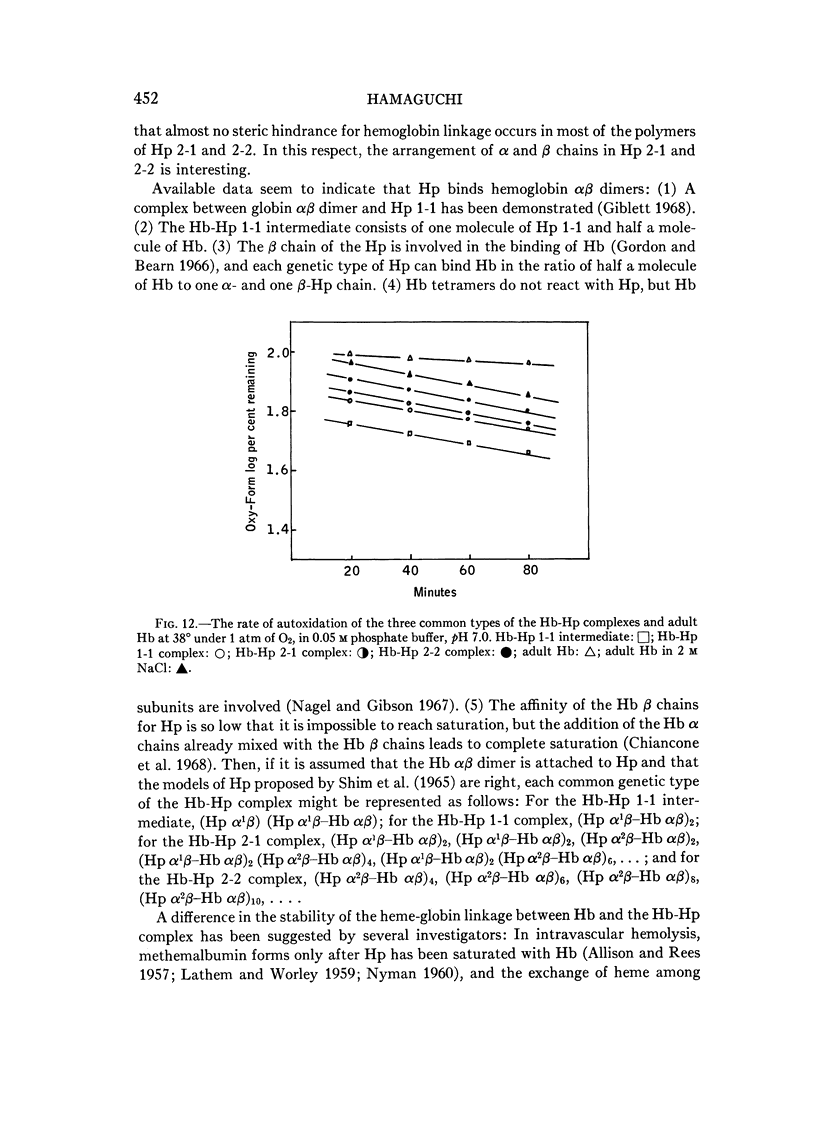
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., REES W. A. The binding of haemoglobin by plasma proteins (haptoglobins); its bearing on the renal threshold for haemoglobin and the aetiology of haemoglobinuria. Br Med J. 1957 Nov 16;2(5054):1137–1143. doi: 10.1136/bmj.2.5054.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. A., Dixon G. H. Amino-acid sequence of alpha chains of human haptoglobins. Nature. 1968 May 25;218(5143):736–741. doi: 10.1038/218736a0. [DOI] [PubMed] [Google Scholar]
- Brunori M., Alfsen A., Saggese U., Antonini E., Wyman J. Studies on the oxidation-reduction potentials of heme proteins. VII. Oxidation-reduction equilibrium of hemoglobin bound to haptoglobin. J Biol Chem. 1968 Jun 10;243(11):2950–2954. [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- CONNELL G. E., DIXON G. H., SMITHIES O. Subdivision of the three common haptoglobin types based on 'hidden' diffrences. Nature. 1962 Feb 3;193:505–506. doi: 10.1038/193505a0. [DOI] [PubMed] [Google Scholar]
- Chiancone E., Alfsen A., Ioppolo C., Vecchini P., Agrò A. F., Wyman J., Antonini E. Studies on the reaction of haptoglobin with haemoglobin and haemoglobin chains. I. Stoichiometry and affinity. J Mol Biol. 1968 Jul 14;34(2):347–356. doi: 10.1016/0022-2836(68)90258-1. [DOI] [PubMed] [Google Scholar]
- Cleve H., Gordon S., Bowman B. H., Bearn A. G. Comparison of the tryptic peptides and amino acid composition of the beta polypeptide chains of the three common haptoglobin phenotypes. Am J Hum Genet. 1967 Nov;19(6):713–721. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R. Recent advances in heptoglobin and transferrin genetics. Bibl Haematol. 1968;29:10–20. doi: 10.1159/000384590. [DOI] [PubMed] [Google Scholar]
- Gordon S., Bearn A. G. Hemoglobin binding capacity of isolated heptoglobin polypeptide chains. Proc Soc Exp Biol Med. 1966 Mar;121(3):846–850. doi: 10.3181/00379727-121-30905. [DOI] [PubMed] [Google Scholar]
- Gordon S., Cleve H., Bearn A. G. An improved method of preparing haptoglobin polypeptide chains using guanidine hydrochloride. Proc Soc Exp Biol Med. 1968 Jan;127(1):52–59. doi: 10.3181/00379727-127-32619. [DOI] [PubMed] [Google Scholar]
- HEIMBURGER N., HEIDE K., HAUPT H., SCHULTZE H. E. BAUSTEINANALYSEN VON HUMANSERUMPROTEINEN. Clin Chim Acta. 1964 Oct;10:293–307. doi: 10.1016/0009-8981(64)90059-2. [DOI] [PubMed] [Google Scholar]
- LATHEM W., WORLEY W. E. The distribution of extracorpuscular hemoglobin in circulating plasma. J Clin Invest. 1959 Mar;38(3):474–483. doi: 10.1172/JCI103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B., NYMAN M. Studies on the serum haptoglobin level in hemoglobinemia and its influence on renal excretion of hemoglobin. Blood. 1957 Jun;12(6):493–506. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemberg R., Legge J. W., Lockwood W. H. Coupled oxidation of ascorbic acid and haemoglobin: Formation and properties of choleglobin. Biochem J. 1941 Mar;35(3):328–338. doi: 10.1042/bj0350328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. K., CONNELL G. E., PERT J. H. The role of haptoglobin in the clearance and distribution of extracorpuscular hemoglobin. Blood. 1961 Jan;17:45–53. [PubMed] [Google Scholar]
- NAKAJIMA H., TAKEMURA T., NAKAJIMA O., YAMAOKA K. STUDIES ON HEME ALPHA-METHENYL OXYGENASE. I. THE ENZYMATIC CONVERSION OF PYRIDINE-HEMICHROMOGEN AND HEMOGLOBIN-HAPTOGLOBIN INTO A POSSIBLE PRECURSOR OF BILIVERDIN. J Biol Chem. 1963 Nov;238:3784–3796. [PubMed] [Google Scholar]
- NYMAN M. On plasma proteins with heme or hemoglobin binding capacity. Scalpel (Brux) 1960;12:121–130. [PubMed] [Google Scholar]
- NYMAN M. Serum hatoglobin; methodological and clinical studies. Scand J Clin Lab Invest. 1959;11(Suppl 39):1–169. [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H. Kinetics of the reaction of carbon monoxide with the hemoglobin-haptoglobin complex. J Mol Biol. 1966 Dec 28;22(2):249–255. doi: 10.1016/0022-2836(66)90130-6. [DOI] [PubMed] [Google Scholar]
- Naito S. Type-dependent difference in the clearance rate of hemoglobin-haptoglobin complexes from the blood stream. Jinrui Idengaku Zasshi. 1967 Dec;12(3):177–186. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- SMITH H., EDMAN P., OWEN J. A. N-Terminal amino-acids of human haptoglobins. Nature. 1962 Jan 20;193:286–287. doi: 10.1038/193286a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O., CONNELL G. E., DIXON G. H. Chromosomal rearrangements and the evolution of haptoglobin genes. Nature. 1962 Oct 20;196:232–236. doi: 10.1038/196232a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O., CONNELL G. E., DIXON G. H. Inheritance of haptoglobin subtypes. Am J Hum Genet. 1962 Mar;14:14–21. [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O., WALKER N. F. Genetic control of some serum proteins in normal humans. Nature. 1955 Dec 31;176(4496):1265–1266. doi: 10.1038/1761265a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O., WALKER N. F. Notation for serum-protein groups and the genes controlling their inheritance. Nature. 1956 Sep 29;178(4535):694–695. doi: 10.1038/178694a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P. K., Doty P. The optical rotatory properties of the beta-configuration in polypeptides and proteins. Proc Natl Acad Sci U S A. 1966 Apr;55(4):981–989. doi: 10.1073/pnas.55.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Beck W. S. Peroxidase activity of hemoglobin and its subunits: effects thereupon of haptoglobin. Biochim Biophys Acta. 1967 Oct 23;147(2):324–333. doi: 10.1016/0005-2795(67)90410-2. [DOI] [PubMed] [Google Scholar]
- Smithies O., Connell G. E., Dixon G. H. Gene action in the human haptoglobins. I. Dissociation into constituent polypeptide chains. J Mol Biol. 1966 Nov 14;21(2):213–224. doi: 10.1016/0022-2836(66)90092-1. [DOI] [PubMed] [Google Scholar]
- Waks M., Alfsen A. Structural studies of haptoglobins. I. Hydrogen ion equilibria and optical rotatory dispersion of Hp 1-1 and Hp 2-2. Arch Biochem Biophys. 1966 Feb;113(2):304–314. doi: 10.1016/0003-9861(66)90191-3. [DOI] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]