Abstract
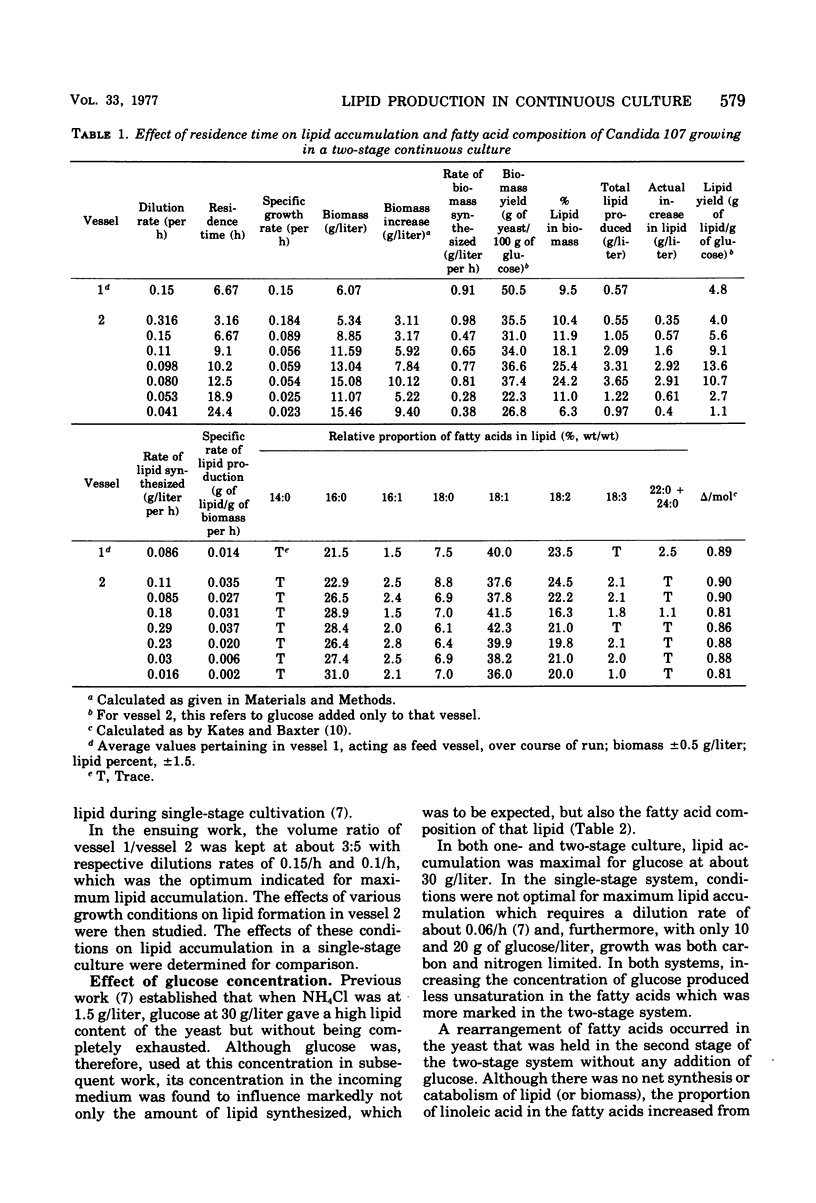
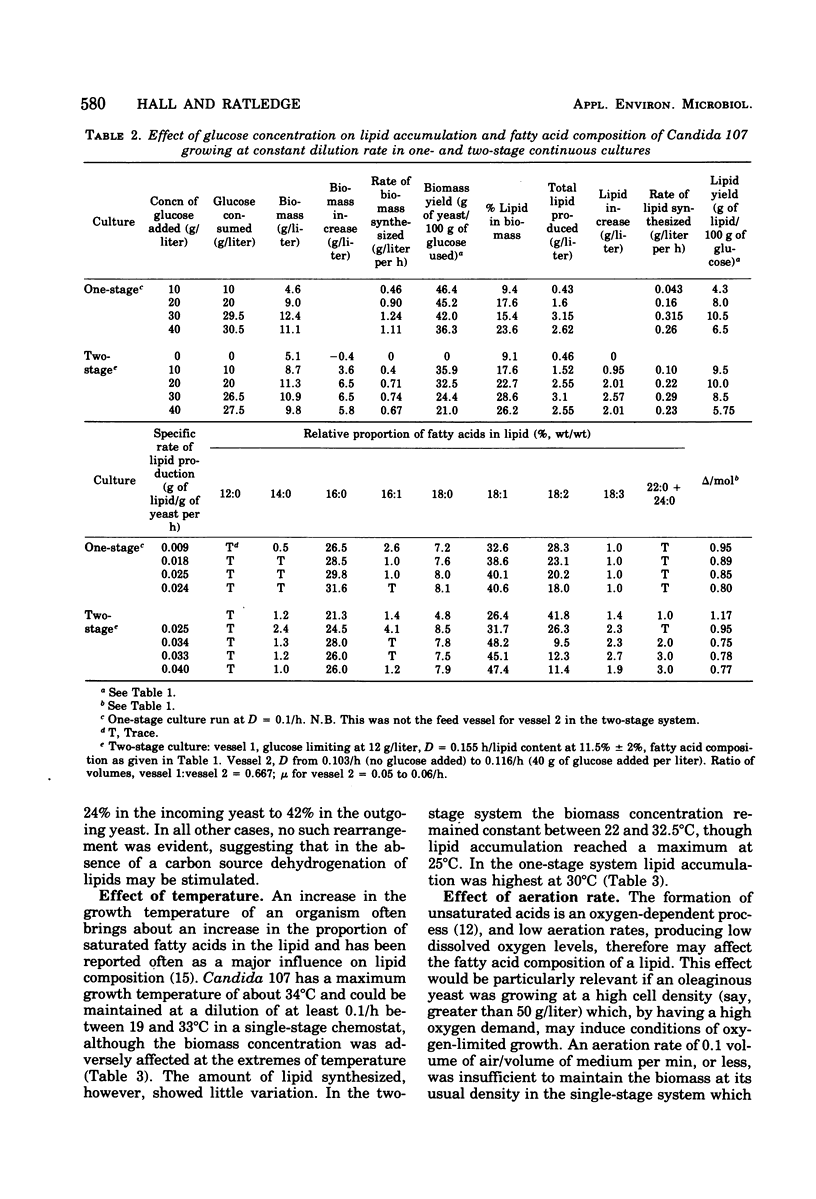
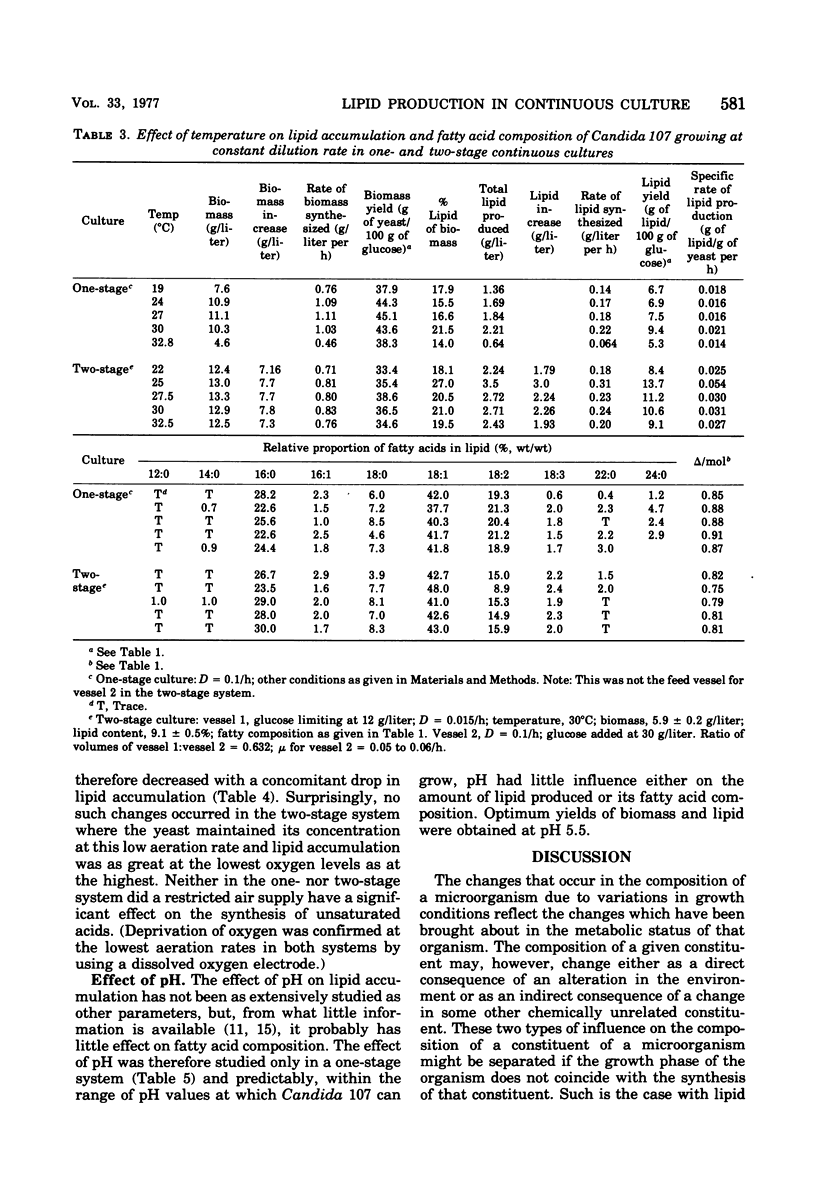
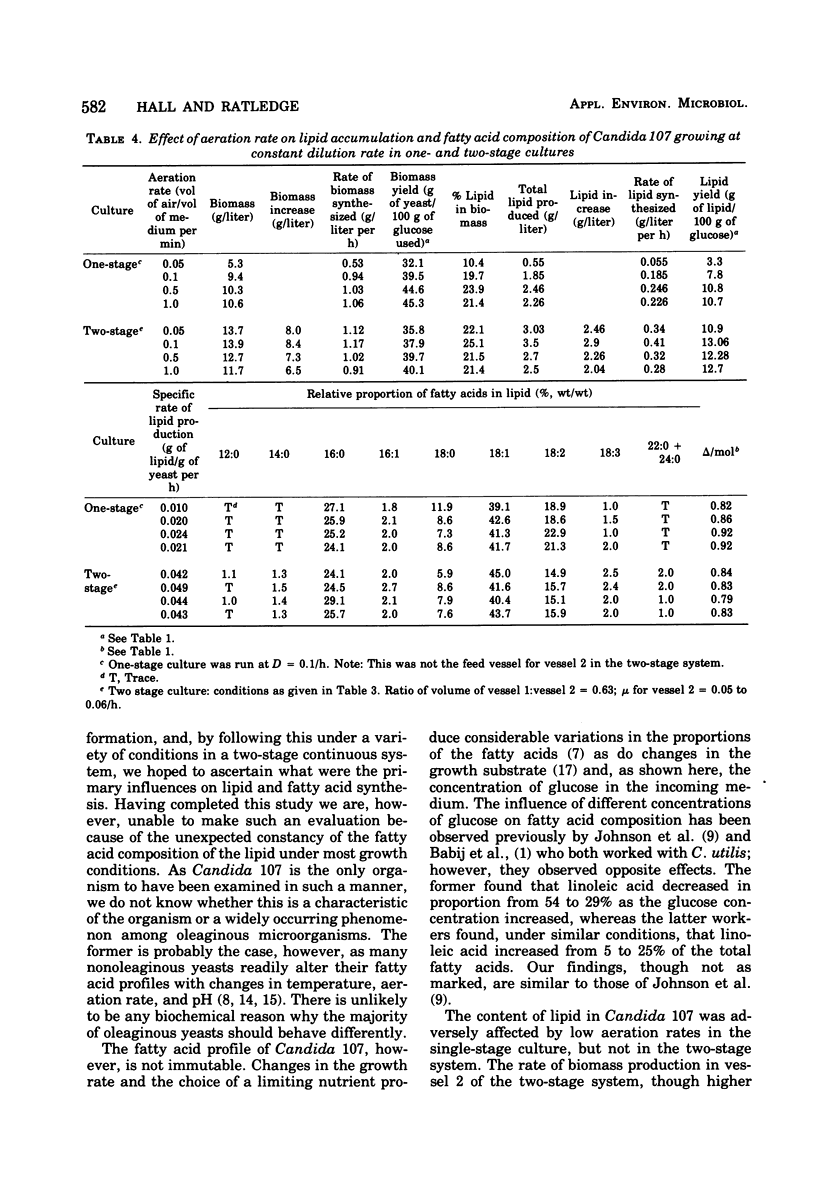
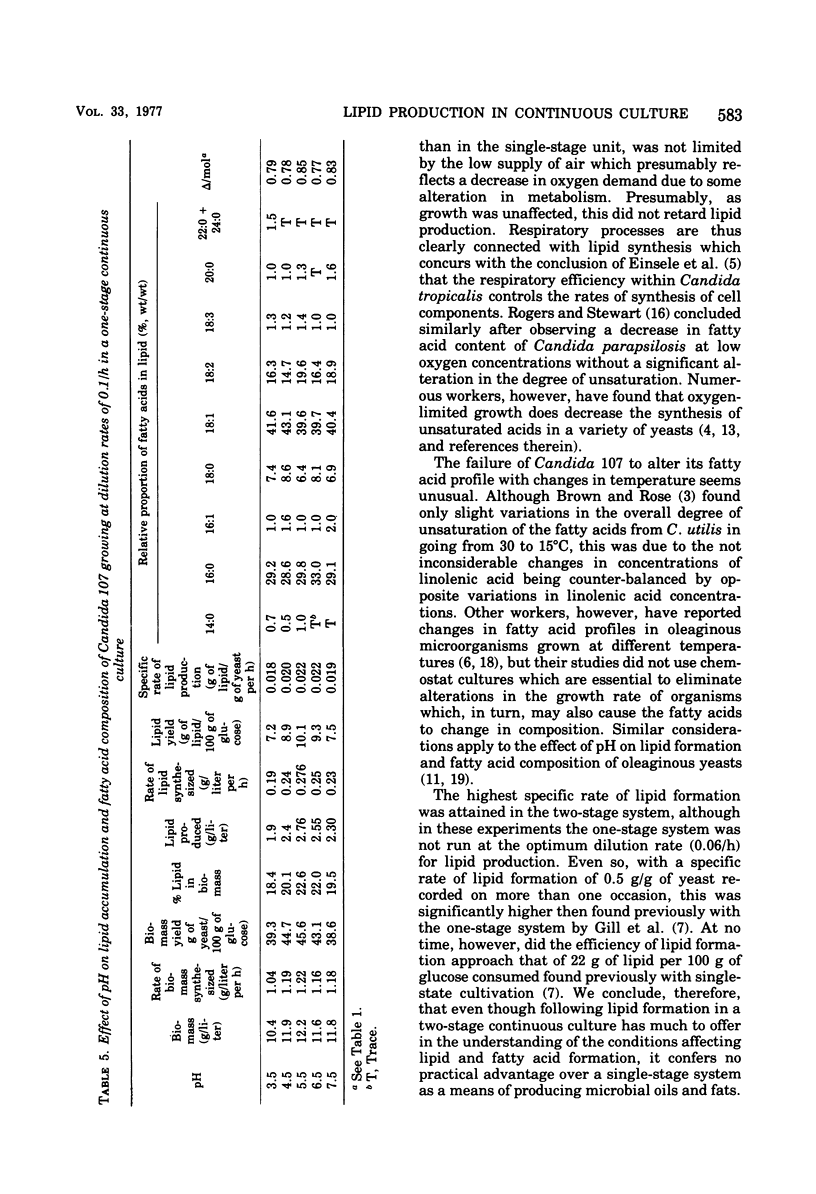
Lipid accumulation and fatty acid composition in Candida 107 have been studied using a two-stage continuous culture system in which the first vessel was run under carbon-limited conditions and then the entire output was passed into a second vessel, where lipid accumulation was stimulated by adding only glucose. Maximum lipid accumulation (28% of yeast [dry weight]) occurred for a volume ratio of vessel 1 to vessel 2 of 3:5, with 30 g of glucose per liter being added to vessel 2 operated at 25°C with an aeration rate of between 0.1 and 1.0 volume of air/volume of medium per min. Although the maximum specific rate of lipid formation (0.05 g of lipid/g of yeast per h) was higher than in a nitrogen-limited, single-stage system, the efficiency of lipid formation was much less and never exceeded 14 g of lipid produced per 100 g of glucose consumed. The fatty acid composition was not significantly altered in either the two-stage or single-stage culture (nitrogen-limited) systems by changes in growth temperature (from 19 to 33°C) or aeration rates (0.05 to 1.0 volume of air/volume of medium per min); or, in the two-stage system, by changes in the residence time of the yeast in the second vessel (from 3.2 to 24.4 h), or, in the single-stage system, by changes in pH (from 3.5 to 7.5). Only when the concentration of glucose entering vessel 2 of the two-stage system was less than 30 g/liter did significant changes in the fatty acids occur. Thus, although a two-stage continuous culture system allows lipid accumulation to be separated from the growth phase, it offers no practical advantages over a single-stage system as a means of producing microbial oils and fats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babij T., Moss F. J., Ralph B. J. Effects of oxygen and glucose levels on lipid composition of yeast Candida utilis grown in continuous culture. Biotechnol Bioeng. 1969 Jul;11(4):593–603. doi: 10.1002/bit.260110407. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Johnson B. Influence of oxygen tension on the physiology of Saccharomyces cerevisiae in continuous culture. Antonie Van Leeuwenhoek. 1971;37(4):477–487. doi: 10.1007/BF02218518. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci M. C., Belloni G., Gianani L. Oxygen pressure, fatty acid composition and ergosterol level in Rhodotorula gracilis. Arch Microbiol. 1975 Sep 30;105(1):17–20. doi: 10.1007/BF00447106. [DOI] [PubMed] [Google Scholar]
- Einsele A., Fiechter A., Knöpfel H. P. Respiratory activity of Candida tropicalis during growth on hexadecane and on glucose. Arch Mikrobiol. 1972;82(3):247–253. doi: 10.1007/BF00412196. [DOI] [PubMed] [Google Scholar]
- Enebo L., Iwamoto H. Effects of cultivation temperature on fatty acid composition in Rhodotorula gracilis. Acta Chem Scand. 1966;20(2):439–443. doi: 10.3891/acta.chem.scand.20-0439. [DOI] [PubMed] [Google Scholar]
- Gill C. O., Hall M. J., Ratledge C. Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose in single-stage continuous culture. Appl Environ Microbiol. 1977 Feb;33(2):231–239. doi: 10.1128/aem.33.2.231-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Nelson S. J., Brown C. M. Influence of glucose concentration on the physiology and lipid composition of some yeasts. Antonie Van Leeuwenhoek. 1972;38(2):129–136. doi: 10.1007/BF02328084. [DOI] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- Kessell R. H. Fatty acids of Rhodotorula gracilis: fat production in submerged culture and the particular effect of pH value. J Appl Bacteriol. 1968 Jun;31(2):220–231. doi: 10.1111/j.1365-2672.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Klein H. P., Volkmann C. M. Factors affecting the palmitoyl-coenzyme A desaturase of Saccharomyces cerevisiae. J Bacteriol. 1975 Nov;124(2):718–723. doi: 10.1128/jb.124.2.718-723.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Konttinen K., Suomalainen H. Neutral lipids in the cells and cell envelope fractions of aerobic baker's yeast and anaerobic brewer's yeast. Chem Phys Lipids. 1975 Feb;14(1):15–32. doi: 10.1016/0009-3084(75)90012-2. [DOI] [PubMed] [Google Scholar]
- Rattray J. B., Schibeci A., Kidby D. K. Lipids of yeasts. Bacteriol Rev. 1975 Sep;39(3):197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Energetic efficiency and maintenance. Energy characteristics of Saccharomyces cerevisiae (wild type and petite) and Candida parapsilosis grown aerobically and micro-aerobically in continuous culture. Arch Microbiol. 1974;99(1):25–46. doi: 10.1007/BF00696220. [DOI] [PubMed] [Google Scholar]
- Zalashko M. V., Andreevskaia V. D., Obraztsova N. V. Vliianie temperatury kul'tivirovaniia na zhirnokislotnyi sostav lipidov drozhzhei, vyrazhchivaemykh na gidrolizatakh torfa. Prikl Biokhim Mikrobiol. 1972 Nov-Dec;8(6):891–895. [PubMed] [Google Scholar]


