Abstract
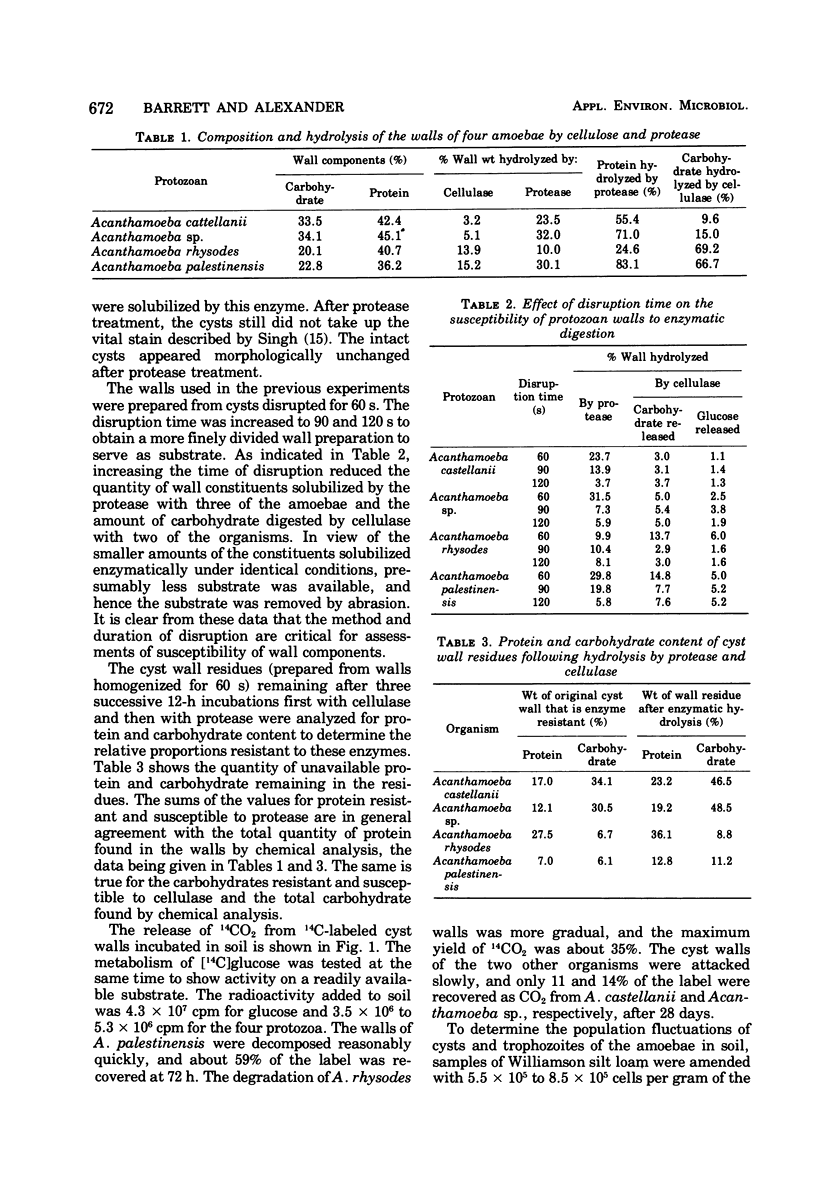
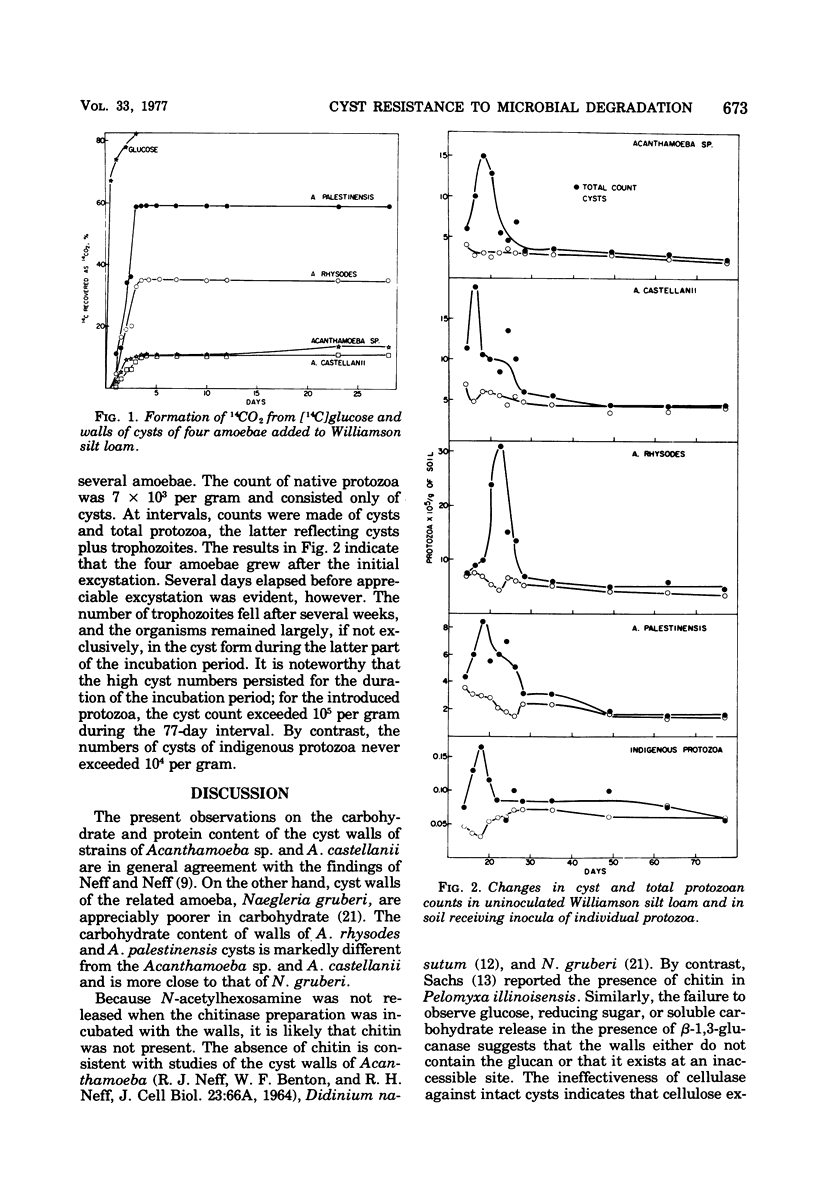
Cyst walls of Acanthamoeba rhysodes, A. palestinensis, A. castellanii, and one other strain of Acanthamoeba contain 36 to 45% protein and 20 to 34% carbohydrate. More than half of the protein in the walls of A. palestinensis, A. castellanii and Acanthamoeba sp. is accessible to and hydrolyzed by protease, and 67 to 69% of the carbohydrate of A. palestinensis and A. rhysodes walls is hydrolyzed by cellulase. The extent of hydrolysis of walls of the other amoebae by these enzymes is appreciably less, and chitinase and β-1,3-glucanase have no detectable effect. Protease solubilizes 10% or less of the weight of intact cysts, and no solubilization is observed with cellulase. Walls of A. palestinensis are extensively degraded in soil, the activity is less with A. rhysodes, and little attack on the other amoebae occurs. When added to soil, the protozoa excyst and grow for short periods, the trophozoites then die, and chiefly cysts persist thereafter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAND R. N. Nutritional and related biological studies on the free-living soil amoeba, Hartmannella rhysodes. J Gen Microbiol. 1959 Aug;21:80–95. doi: 10.1099/00221287-21-1-80. [DOI] [PubMed] [Google Scholar]
- BRZEZINSKA-DUDZIAK B. Oznaczanie ilości ameb w glebie wg zmodyfikowanej metody Singh'a. Acta Microbiol Pol. 1954;3(2):121–124. [PubMed] [Google Scholar]
- Danso S. K., Keya S. O., Alexander M. Protozoa and the decline of Rhizobium populations added to soil. Can J Microbiol. 1975 Jun;21(6):884–895. doi: 10.1139/m75-131. [DOI] [PubMed] [Google Scholar]
- Habte M., Alexander M. Protozoa as agents responsible for the decline of Xanthomonas campestris in soil. Appl Microbiol. 1975 Feb;29(2):159–164. doi: 10.1128/am.29.2.159-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff R. J., Neff R. H. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51–81. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Singh B. N., Datta T., Dutta G. P. Factors inducing excystation in free-living amoebae. Indian J Exp Biol. 1971 Jul;9(3):350–357. [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth J. M., Kahn A. J. Isolation and preliminary chemical analysis of the cyst wall of the amoeba-flagellate Naegleria grüberi. J Bacteriol. 1967 Oct;94(4):1272–1274. doi: 10.1128/jb.94.4.1272-1274.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


