Abstract
An intermolecular intercalation of base pairs was found at the CA step in the I222 crystal structure of the RNA·DNA hybrid, r(CAAAGAAAAG)·d(CTTTTCTTTG), which contains two-thirds of the polypurine tract sequence of HIV-1 with a substitution of cytosine for the initial adenine. This sequence crystallized in both P212121 and I222 space groups, with an rms difference of only 0.63 Å between residues 3 to 18 of the two forms. P212121 and I222 helices are both A-like, but intercalation occurs only in the I222 crystal form. The present structure shows bases stacked in parallel rather than perpendicular as in intercalated DNA (I-DNA). The base intercalation is also different from zipper-like meshing of bases seen in the center of the crystal structure of d(GCGAAAGCT), which does not have Watson–Crick base pairing. The base-step intercalation seen here is reminiscent of domain swapping in proteins; therefore, we call this phenomenon “base-pair swapping.” It involves a highly mobile CA step and seems to be sequence-specific and electrostatically stable without disrupting Watson–Crick interactions. It also exhibits a large rise concurrent with unwinding of the helix (low twist). We present a base-pair swapping dimer in nucleic acids.
Keywords: RNA·DNA polypurine tract, DNA intercalation
Nucleic acids have been known to adopt various deformations besides the established B, A, and Z helices. DNA can be intermediate between two types of helices (1, 2) and can form triplexes (3) and also structures such as the G-DNA tetraplexes formed by guanine-rich sequences (4–6). Four-stranded tetraplexes are also found in the guanine-rich repeats of RNA (7, 8).
Intercalation by insertion of small aromatic rings between base pairs has been known since the early 1960s (9). Most intercalators such as ethidium and acridine are nonspecific DNA intercalators (10), but some metallo-intercalators such as the rhodium intercalator, Δ-α-[Rh[(R,R)-Me2trien]phi]3+ [where (R,R)-Me2trien is 2R,9R-diamino-4,7-diazadecane] (11) are known to be sequence-specific.
Here we present an RNA·DNA hybrid intermolecular intercalation occurring between two duplexes (Fig. 1a). This type of intercalation resembles domain swapping in proteins (Fig. 1b), which was first introduced to describe a dimer of diphtheria toxin in which two or more protein chains exchange identical domains to form an oligomer (12).
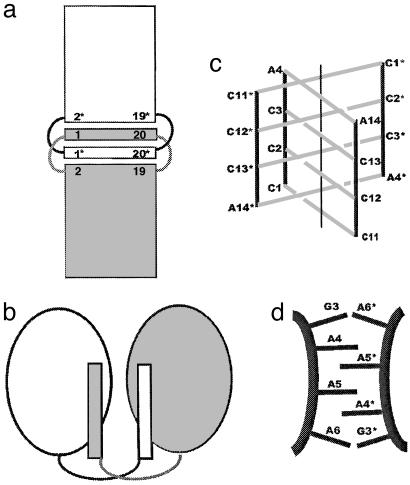
Fig. 1.
Intercalation and domain swapping in nucleic acids and proteins. (a) Schematic diagram of this intercalated RNA·DNA structure. Helices stack head to head. Base pair 1*·20* of one helix intercalates between base pairs 1·20 and 2·19 of the other. Similarly, base pair 1·20 intercalates between base pairs 1*·20* and 2*·19* (* indicates symmetry-related molecule). (b) Schematic of 3D domain swapping as seen in proteins. (c) The central I-DNA motif region of the crystal structure of d(CCCAAT). Two strands are in the asymmetric unit, and an additional duplex (marked with asterisks) completes the four-stranded intercalated motif of the molecule. C·C base pairing is extended to A·A base pairs in this intercalation. (d) The schematic diagram of the zipper-like motif found in the middle of the crystal structure of d(GCGAAAGCT) with alternate intercalations of unpaired adenines and doubling of the interspacing found in this region.
A different type of DNA intercalation, known as I-DNA or the I-motif structure, has been found in a single strand containing four cytidine repeats (13), by association of two strands containing two cytidine repeats (14), or four strands containing a cytidine-rich stretch (15–18) found especially in centromeric and telomeric regions (19). I-DNA involves a systematic base intercalation (20) formed by two parallel strands holding together and interdigitating into each other (Fig. 1c).
DNA can self-intercalate via its base pairs. A zipper-like DNA structure with intercalated bases stacking on each other instead of forming Watson–Crick G·C and A·T base pairs was first modeled in 1983 (21). Detailed structural information on this structure has recently been observed crystallographically (Fig. 1d) showing the unusual zipper-like structure of d(GCGAAAGCT)·d(T*C*G*A*-A*A*G*C*G*) (with one strand per asymmetric unit; asterisks denote a crystallographically related strand). In a central segment of four adenine residues flanked by two sheared G·A mismatches (G3·A6* and A6·G3*) the adenines were not base-paired but instead were intercalated (ref. 22 and Fig. 1d). Such zipper-like motifs have also been demonstrated by unconstrained nanosecond molecular dynamics (23).
Base stacking by self-intercalation has also been found in RNA. In yeast tRNAPhe, the UA base step of 8–9 close to the D stem is spread ≈6.5 Å apart in the vertical direction rather than the normal 3.9 Å. In between the two bases U8 and A9, C13 stacks on both bases and ties residues of 8 and 9 to the D stem by hydrogen bonding between C13-N4 and the phosphate oxygen of A9 (24).
In the RNA·DNA hybrid structure reported here the dimer formation occurs at the outermost CA step of the I222 crystal form of an RNA·DNA hybrid designed as the first two-thirds of the HIV-1 polypurine tract: r(CAAAGAAAAG)·d(CTTTTCTTTG). Cytosine was substituted for adenine at the 5′ end to prevent fraying of the helix. Although this sequence crystallized in both P212121 and I222 space groups, only I222 intercalates (ref. 25 and M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data).
Materials and Methods
Oligonucleotide Synthesis and Purification. The RNA decanucleotide CAAAGAAAAG was purchased from Yale University (Keck Oligonucleotide Synthesis Facility, New Haven, CT) and purified by gel electrophoresis with gels 50 × 30 × 3 cm thick. This purification procedure was crucial in obtaining a very pure RNA strand for crystallization. The DNA strand d(CTTTTCTTTG) was synthesized by the solid-phase phosphoramidate method on an Eppendorf ECOSYN D300 synthesizer. After detritylating the 5′-dimethoxy group, various single-stranded oligonucleotides were separated from the decamer sequence on a DE52 (Whatman) column as reported by Han et al. (26).
Crystallization and Data Collection. The I222 crystal of the RNA·DNA hybrid was grown by the sitting-drop vapor diffusion method at room temperature from Mg(OAc)2, spermidine, n-octyl-β-d-glucoside, 2,4-methyl-pentanediol, and sodium cacodylate at pH 5.8 (M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data). The droplet containing low Mg(OAc)2 (5.14 mM) produced I222 crystals, whereas P212121 crystals were formed with 11.94 mM of either Mg(OAc)2 or Ca(OAc)2. However, the lower pH seems to be more critical for I222 crystals, which formed in the Mg(OAc)2 only at pH 5.8.
Unlike P212121 crystals, which diffracted to high resolution (ref. 25 and M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data), the I222 crystal only diffracted to 2.38 Å on a R-AXIS IV imaging plate at –180°C by using CuKα radiation. X-ray data were processed by using the programs denzo and scalepack (27). Crystal data are shown in Table 1.
Table 1. Crystal data and refinement statistics for r(CAAAGAAAAG)/d(CTTTTCTTTG).
| Crystal data | |
| Space group | 1222 |
| Unit cell parameters, Å | a = 45.234; b = 46.779; c = 56.560 |
| Data collection | |
| Resolution, Å | 2.38 |
| No. of unique reflections | 2,814 |
| Completeness, % | 98.9 |
| Rsym,* % | 5.3 |
| Refinement | |
| Rcryst (Rfree) | 0.223 (0.251) [F ≥ 2σ(F)] |
| 0.238 (0.264) [F ≥ 0σ(F)] | |
| rms deviations | |
| Bonds, Å | 0.004 |
| Angles, ° | 0.82 |
Rsym = Σ|I - 〈I〉|/ΣI.
Structure Solution and Refinement. The structure was solved by molecular replacement (MR) with the program epmr (28). Initially, the high-resolution crystal structure of the P212121 RNA·DNA hybrid of the same sequence (ref. 25 and M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data) was used as a search molecule for the MR method, testing both I212121 and I222 space groups. The best MR solutions were at R = 0.432 with I222 [correlation coefficient (cc) = 0.580] and R = 0.503 with I212121 (cc = 0.407). I222 with the lower R factor was chosen as the correct space group.
The best solution (R = 0.432) for I222 was significantly stronger than the second solution at R = 0.529 (cc = 0.400), and refinement was begun with the former solution by using the program x-plor (29) followed by cns (30). After rigid-body and positional refinement in x-plor, the R factor dropped to 0.350 (Rfree = 0.378). At this stage, 2 Fo – Fc and Fo – Fc maps were calculated. The first two sugars and their phosphate backbone then were adjusted based on the 2 Fo – Fc and Fo – Fc maps, but with difficulty, because the phosphate backbone and sugar density were poor in this region. After simulated annealing and conjugate gradient least-square refinement, the R factor was 0.317 (Rfree = 0.357). However, the R factor did not improve after further refinement.
Hence, it was necessary to check the accuracy of the MR solution. epmr was re-run by using the same model but omitting the problem area: bases 1 and 2 and 19 and 20. This run provided exactly the same solution as before at R = 0.432 (cc = 0.580), implying that the solution was correct.
At this stage, Fo – Fc and 2 Fo – Fc omit maps were recalculated, and residues 1 and 2 and 19 and 20 were remodeled into the density. Surprisingly, the 2 Fo – Fc map without phase bias showed that the phosphate backbone was extended, creating a gap. By intercalating a symmetry hybrid molecule packed head to head into this density, the gap was filled (Fig. 2). The electron density of the DNA strand at residues 19 and 20 was better defined than that of steps 1 and 2 in the RNA strand. The average B factors of residues 1 and 2 (49.68 Å2) were slightly higher than those of residues 19 and 20 (35.45 Å2).
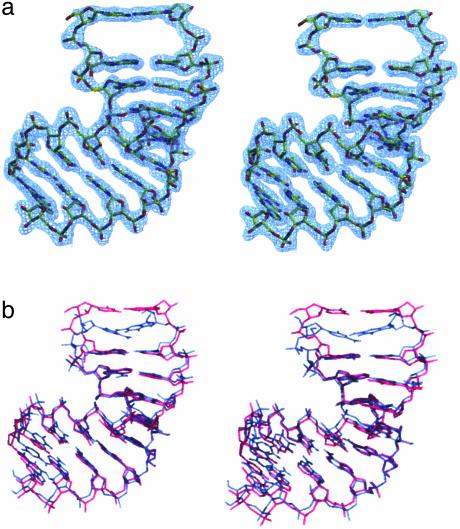
Fig. 2.
2 Fo – Fc and Fo – Fc omit maps calculated by omitting the problem region at base pairs 1·20 and 2·19. Red represents the 3σ level in the Fo – Fc map, and blue represents the 1σ level in the 2 Fo – Fc map. The intercalation bases 1* and 2* are symmetry mates of residues 1 and 2.
This structure solution was reconfirmed by running MR again with epmr with a different but related RNA·DNA hybrid structure, d(CTCCTCTTC)·r(GAAGAGGAG) (31), as a search model. This molecule contained a bulge that was removed from the search model. epmr provided the same clear solution as before, with R = 0.491 (cc = 0.443).
Final refinement was performed with cns by using bulk solvent correction with the cross-validated maximum-likelihood approach incorporated in simulated annealing refinement (32) and with unrestrained sugar conformations for residues 1 and 2 and 19 and 20. At 10- to 2.38-Å resolution, the 2σ R factor was 0.223 (Rfree = 0.251), and the R factor for all the data was 0.238 (Rfree = 0.264). The final structure contains 414 nucleic acid atoms and 66 water molecules.
Results and Discussion
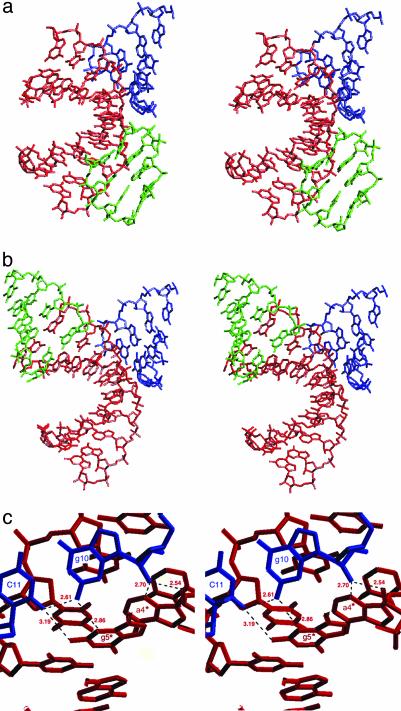
Overall Structure. Fig. 3a shows the overall structure of the I222 RNA·DNA hybrid. One of its striking features is a large gap between base pairs 1·20 and 2·19. This gap is filled by residue 1*·20* of a twofold symmetry-related molecule. As a result, base pairs are swapped at the junction of two molecules. The electron densities shown in Fig. 3a are reasonable and as continuous in the intercalated regions as in the helix as a whole. Overall, this I222 structure exhibits an A-like conformation. The P212121 crystals formed from different crystallization conditions (see Materials and Methods)also exhibit an A-like helix conformation, but no intercalation occurs. The rms difference between the I222 and P212121 structures is 1.32 Å for all residues and 0.63 Å for residues 3–18. The main difference (1.38 Å) occurs at the intercalation region of the first CA step (1 and 2 and 19 and 20) (Fig. 3b).
Fig. 3.
(a) Stereo views of the 2 Fo – Fc electron-density map contoured at the 1σ level. The RNA·DNA hybrid duplex with rC1·dG20 at the top of the helix. (b) The superposition of the I222 (purple) with the P212121 (blue) structure.
The A-like wide minor groove in the I222 hybrid varies in width from 8.8 to 10.3 Å (Fig. 4). The minor groove width in the P212121 form of the RNA·DNA hybrid is a comparable 8.5–10.7 Å. In contrast, the DNA·DNA helix d(CAAAGAAAAG)·d(CTTTTCTTTG), analogous to our RNA·DNA, crystallized in space group C2 with a typical B-helix conformation exhibiting a narrow minor groove width (ref. 26 and Fig. 4).
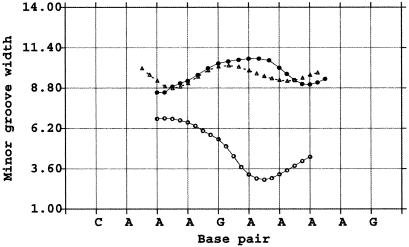
Fig. 4.
Comparison of the minor groove widths between RNA·DNA and DNA·DNA in CAAAGAAAAG helices. Open triangles (I222 form) and filled circles (P212121 form) represent the RNA·DNA hybrids. Open circles represent the DNA·DNA structure. The DNA·DNA shows the B-helix property of a narrow minor groove width, whereas the RNA·DNA hybrids show A-like properties. The P212121 structure shows a relatively wider minor groove width than the I222 hybrid, especially in the abutting packing region. The minor groove width was calculated by curves (33). This program added three intermediate points between base steps to create a smooth curve.
Helical Parameters. Overall helical twist, slide, and X-displacement of both the I222 and P212121 forms are A-like (Table 2). If the intercalation step is omitted, mean helical twist is almost the same in the I222 and P212121 forms.
Table 2. Average helical parameters.
| Helical twist, ° | Slide, Å | Xdisp., Å | Rise, Å | Inclination, ° | |
|---|---|---|---|---|---|
| 1222 form (this work) | |||||
| All steps | 29.0 | -0.2 | -3.6 | 3.5 | 2.6 |
| Intercalation CA step | 16.1 | -0.2 | -3.4 | 6.1 | 9.3 |
| Without intercalation | 30.7 | -0.2 | -4.0 | 3.2 | 2.1 |
| Greatest similarity to standard helix | A | A | A | B | B |
| P212121 form (ref. 25) | 31.9 (31.3) | -0.1 (-1.3) | -3.3 (-2.1, -3.6) | 3.0 (2.7) | 5.7 (4.7, 4.4) |
| DNA·DNA structure of same sequence (ref. 26) | 35.5 (35.2) | 0.0 (-0.1) | -0.4 (-2.7) | 3.3 (3.4) | -2.0 (-2.0) |
| Region IV* of RT-RNA·DNA (ref. 34) | 32.2 | 0.1 | -2.0 | 3.2 | -3.9 |
| A-DNA (ref. 35) | 31.1 | -1.6 | -4.1 | 2.9 | 12.0 |
| A-RNA (ref. 36) | 33.8 | -1.2 | -3.6 | 2.7 | 17.0 |
| B-DNA (ref. 35) | 36.0 | 0.4 | -0.1 | 3.4 | 2.4 |
| Nogalamycin/CA step† intercalation (ref. 37) | 35.1 | 0.3 | -0.2 | 7.3 | -1.0 |
Values in parentheses were calculated by using FREEHELIX; all others were calculated by using CURVES. X-displacement (Xdisp.) and inclination are calculated separately for base pairs 1-5 and 6-10 in P212121 because of their sensitivity to choice of helix axis in a bent molecule.
Base pairs 14-19 of r(AAGAAA)·d(TTTCTT) of polypurine tract.
Values from two d(TGTACA)·nogalamycin molecules are averaged.
The doubling of the rise at the CA intercalation step (6.1 Å) of the I222 hybrid increases the average rise value for all steps to 3.5 Å along the helix (Table 2). However, the average rise without the intercalation step (3.2 Å) is intermediate between A (2.7–2.9 Å) and B (3.4 Å) helices, more B-like than the P212121 RNA·DNA hybrid (2.7 Å).
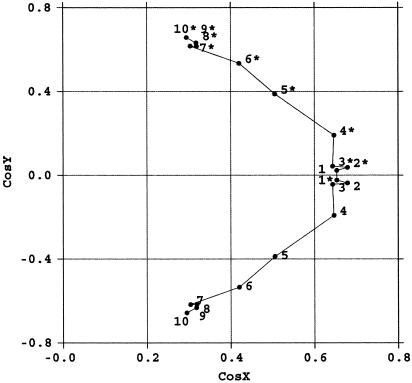
Helix Bending. Fig. 5 shows the normal vector plot for the intercalated 20-bp helix dimer consisting of r(CAAAGAAAAG)·d (CTTTTCTTTG) and r(C*A*A*A*G*A*A*A*A*G*)·d (C*T*T*T*T*C*T*T*T*C*) (asterisks indicate a neighboring molecule). Each helix is generally straight at its initial intercalation site (C1-A2), bent at the A4-G5-A6 steps, and straight again at the end of the A6-A7-A8-A9 region (for a more extensive explanation of normal vector plots see figures 4–6 of ref. 38). The relatively small roll angles, except at the AGA step in both I222 and P212121 forms, are consistent with this helix bend at the AGA step.
Fig. 5.
Normal vector plot for intercalated 20-bp helix dimer. Helices are straight at the end and bent at the AGA steps (for a more extensive explanation of normal vector plots, see figures 4–6 of ref. 38). Notice that intercalation occurs in the middle of the two straight regions.
A-Like Packing Interactions. In addition to the interhelix intercalation unique to this structure (Fig. 6), the familiar A-like “base pair into minor groove” packing is seen at the other end of the helix (Fig. 7). Watson–Crick hydrogen bonding occurs between C(11)-O2 and G(10)-N2, and the C(11)-O2 is also involved in an intermolecular hydrogen bond with G(5)*-N2. N2 of G(10) is involved in an intermolecular hydrogen bond with N3 of G(5)* as shown in Fig. 7c. The O2′ of G(10) makes two intermolecular hydrogen bonds with O2′ and N3 of A(4)*, with bond lengths 2.54 and 2.70 Å, respectively. This provides quadruple, G5*·(G10·C11)·A4*, interactions that reinforce stability in this region (Fig. 7c).
Fig. 6.
(a) Two intercalated RNA·DNA hybrids stack head to head. Helical axes were drawn by curves.(b) Ninety-degree rotation view of the hybrid. The central intercalation region is colored in red, rC1·dG20 and blue, rC1*·dG20*.
Fig. 7.
Intermolecular contacts observed in the P212121 (a) and I222 (b) structures. (a) In the P212121 structure, two neighboring molecules (shown as blue and green) pack into the minor groove of the molecule (red), which is typical A-like packing. (b) The I222 structure has a new intercalation (green) as well as an A-like abutting packing (blue). (c) Close up view of the abutting interactions in the I222 structure with the hydrogen bonds indicated.
Intercalated Helix Conformation. The doubling of the rise at the intercalation region is similar to that which occurs when drugs or small molecules intercalate between base pairs (39–45). However, the helix unwinding at the intercalation step in our structure is very different. Our intercalator is not a free aromatic ring but another DNA base pair connected to its backbone chains. To prevent steric clash between backbones of the two helices at the intercalation site, helical twist must be unwound to 16.1° (Table 2). This is unnecessary, and not observed, in a typical drug CA base intercalation step (Table 2).
Backbone torsion angles at the intercalated site are similar to those found in normal A-DNA or A-RNA. Torsion angles of α(331.7°), ζ(280.5°), and ε(236.7°) are similar to that of A-RNA (313°, 281°, and 213°, respectively; ref. 46). However, the base-sugar torsion angle, χ(205.1°), and main chain, β(193.1°), are quite different from those of A-RNA (13° and 175°, respectively). These values for the conformation angles at the intercalation site agree with the Berman model in which intercalation geometry is A-like except for χ and β (46).
Intercalation does not seem to favor any particular sugar conformation, because several sugar conformations have been found in intercalated structures (42–44). In our case, all sugar puckers are C3′-endo except for residue 2 on the RNA strand. Both C3′-endo and C2′-endo conformations were tried at this locus, and although the difference density was weak, the C2′-endo conformation was favored. This sugar switch at residue 2 is also seen in the high resolution P212121 structure (ref. 25 and M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data).
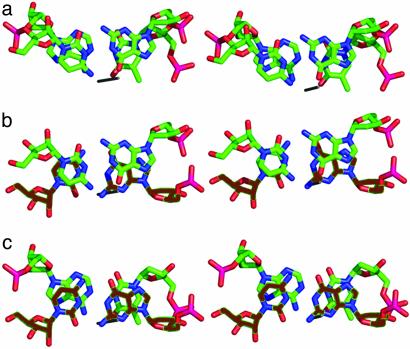
Deformability of the Initial Pyrimidine-Purine CA Step Rather Than the Final Purine-Purine AG Step. Intercalation occurred only in the very flexible pyrimidine-purine CA step but not in the purine-purine AG step at the other end of the helix. Pyrimidine-purine steps are known to be weak and to exhibit relatively little ring–ring overlap between adjacent base pairs (47). They are especially susceptible to large twist and slide deformations and to bending via a positive roll. In contrast, purine-purine steps are stabilized by extensive ring–ring overlap from one base pair to the next, which suggests that CA steps should be more prone to intercalation than AG steps. Intercalation replaces the weaker stacking by a CA step (base pairs 1·20 and 2·19) (Fig. 8a), with a CC* step (base pairs 1·20 and 1*·20*) (Fig. 8b), followed by another C*A step (base pairs 1*·20* and 2·19) (Fig. 8c).
Fig. 8.
Local base-pair stacking without (a) and with (b and c) rC1·dG20 intercalation. (a) CA step: base pairs rC1·G20 (front) and rA2·dT19 (behind). (b) CC* step: base pairs rC1·G20 (front) and 1*·20* (behind; brown). (c) C*A step: base pairs rC1*·dG20* (front; brown) and rA2·dT19 (behind). Note that base-pair overlap is enhanced by intercalation.
Role of Helix Intercalation. By base swapping between neighboring duplexes, the unfavorable amine-amine (N4-N6) stacking in the C(1)-A(2) step is replaced with favorable amine-carbonyl (N4-O2*) and carbonyl-amine (O2*-N6) stacking in C(1)-C(1)* and C(1)*-A(2) steps (Fig. 8 b and c). Also, the O5′ and N4 and N1 of C(1)* are involved in hydrogen bonding with the O1P, N3, and N7 of A(2).
The DNA strand shows a favorable situation as well. The O4-O6, carbonyl-carbonyl stacking in the T(19)-G(20) step is replaced by favorable O4-N2*, carbonyl-amine and amine-carbonyl stacking in the T(19)-G(20)*, and G(20)*-G(20). Thus, favorable O4-N2-O6, carbonyl-amine-carbonyl stacking in T(19)-G(20)*-G(20) occurs by G* intercalation.
Hence, the base-swapped dimer is stabilized both by van der Waals interactions as well as hydrogen bonds at the intercalated region between two molecules.
Comparison of Nucleic Acid Intercalated Structures. As shown in Table 3, our intercalation has similarities as well as striking differences from I-DNA and the zipper-like DNA duplex. All involve intercalation at the CA steps. In the zipper-like DNA duplex, bases are not paired. In I-DNA, although the bases are paired, they are paired by non-Watson–Crick hydrogen bonds; however, ours do show Watson–Crick base-pairing. Our structure resembles 3D domain-swapping structures found in proteins. The identical C·G base pairs were exchanged to form a dimer in the same way that identical domains exchange to form an oligomer, which has been proposed as a mechanism that explains the evolution from monomeric to oligomeric proteins (48).
Table 3. Comparison between nucleic acid intercalated structures.
End-to-End Stacking in RNA·DNA Hybrid. Our base-pair swapping occurs at the end-to-end interaction of the RNA·DNA hybrid. End-to-end packing is uncommon in RNA·DNA structures. In the dominant “A-helix packing” (Table 4 and Fig. 7a), neighboring helices pack against the walls of the minor groove. However, end-to-end stacking has been seen in r(UUCGGGCGCC)·d(GGCGCCCGAA) (52). In this structure, residue 1 stacks on top of residue 20* (head to tail), simulating a continuous helix for two molecules, as in a B-helix. In contrast, residue 1 stacks on top of residue 1* in our molecule. In other words, residues 1 and 1* are packed by twofold symmetry (the twofold center is at the center of 1·20 and 1*·20* residues). The large rise (6.1 Å) and unwinding of the helical twist (16.1°) permit its intercalation.
Table 4. Single crystal structures for RNA·DNA hybrid duplexes.
| Sequence | Structure | Space group | Crystal packing | Ref. |
|---|---|---|---|---|
| r(CAAAGAAAAG)·d(CTTTTCTTTG) | Intercalation | 1222 | Abutting and head-to-head | This work |
| RNA-C2′ endo for 2A, C3′ endo for others | (1 and 1* and 20 and 20*) | |||
| DNA-C3′ endo | ||||
| r(CAAAGAAAAG)·d(CTTTTCTTTG) | A-like | P212121 | Abutting | 25 and M.L.K., L. Lavelle, G.W.H., H.-L. Ng, and R.E.D., unpublished data |
| r(GAAGAAGAG)·d(CTCTTCTTC) | A-like | P61 | Abutting | 49 |
| r(GAAGAGAGAG)·d(CTCCTCTTC) | A-like flipped-out base | 1222 | Abutting and head-to-head, looped-out, bulge interaction | 31 |
| r(GAAGAGAAGC)·d(GCTTCTCTTC) | A-like | P212121 | Abutting | 50 |
| r(GAAGAAGAA)·d(TTCTTBr5CTTC) | A-like | P61 | Abutting | 51 |
| r(UUCGGGCGCC)·d(GGCGCCCGAA) | RNA-C3′ endo | P4322 | Abutting and head-to-tail | 52 |
| DNA-C2′ endo for AA, C3′ endo for others | (1 and 20*, and 20 and 1*) |
Summary
The RNA·DNA hybrid polypurine tract sequence can crystallize in two space groups: P212121, with only A-like packing, and I222, with A-like packing at one end and a previously uncharacterized CA intercalation step at the other. I222 diffracts to only 2.38-Å resolution, whereas the P212121 crystal diffracts to 1.15-Å resolution. A-helix-type packing is stabilized by more hydrogen bonds (Fig. 8c) than end-to-end stacking.
Pyrimidine-purine CA tracts of DNA are involved in recombination, transcription gene conversion, and insertion events and in the enhancement of recombination (53, 54). In the crystal structure of d(ACCGGCGCCACA)·d(TGTGGCGCCGGT), Watson–Crick base pairing in the major groove of (CA)n tracts are disrupted by tilting of the bases, and hydrogen bonds were shifted to the 5′ neighbor of their complement (53).
This CA step is responsible for highly variable stacking patterns such as the I-DNA motif (13–17, 20), the zipper-like structure (22), and base-pair shifting to a tilted stack that disrupts Watson–Crick base pairing (53). We now add base-pair swapping involving a CA step. Investigation of its potential role in biology deserves further study.
Acknowledgments
We thank Thang Kien Chiu for the shelxdna program for plotting helical parameters and Yong Xiong for the discussion about the sugar parameters for nucleic acids in cns refinement. We also thank Ho-Leung Ng and Gail Katsir for helpful discussion and comments in preparing this manuscript and Vivian Yip and Wai-Yin Ng for help with purification of the DNA strand. This work was supported by National Institutes of Health Grant GM-31299 and an Amgen Fellowship (to G.W.H.) from the University of California (Los Angeles) Center for AIDS Research.
Abbreviations: I-DNA, intercalated DNA; MR, molecular replacement; cc, correlation coefficient.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1JB8), and the atomic coordinates have been deposited in the Nucleic Acid Database, Department of Chemistry, Rutgers, The State University of New Jersey, Piscataway (NDB structure ID code AH0019).
References
- 1.Ng, H. L., Kopka, M. L. & Dickerson, R. E. (2000) Proc. Natl. Acad. Sci. USA 92, 10767–10771. [Google Scholar]
- 2.Vargason, J. M., Henderson, K. & Ho, P. S. (2001) Proc. Natl. Acad. Sci. USA 98, 7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, J. R., Woodsworth, M. L., Latimer, L. J. & Morgan, A. R. (1984) Nucleic Acids Res. 12, 6603–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkinson, G. N., Lee, M. P. H. & Neidle, S. (2002) Nature 417, 876–879. [DOI] [PubMed] [Google Scholar]
- 5.Williamson, J. R., Raghuraman, M. K. & Cech, T. R. (1989) Cell 59, 871–880. [DOI] [PubMed] [Google Scholar]
- 6.Sundquist, W. I. & Klug, A. (1989) Nature 342, 825–829. [DOI] [PubMed] [Google Scholar]
- 7.Kim, J., Cheong, C. & Moore, P. B. (1991) Nature 351, 331–332. [DOI] [PubMed] [Google Scholar]
- 8.Deng, J., Xiong, Y. & Sundaralingam, M. (2001) Proc. Natl. Acad. Sci. USA 98, 13665–13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerman, L. S. (1961) J. Mol. Biol. 3, 18–30. [DOI] [PubMed] [Google Scholar]
- 10.Wilson, W. D. & Jones, R. L. (1981) Adv. Pharmacol. Chemother. 18, 177–222. [DOI] [PubMed] [Google Scholar]
- 11.Kielkopf, C. L., Erkkila, K. E., Hudson, B. P., Barton, J. K. & Rees, D. C. (2000) Nat. Struct. Biol. 7, 117–121. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, M. J., Choe, S. & Eisenberg, D. S. (1994) Proc. Natl. Acad. Sci. USA 91, 3127–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroy, J.-L., Guéron, M., Mergny, J.-L. & Helene, C. (1994) Nucleic Acids Res. 22, 1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohozinski, J., Hancock, J. M. & Keniry, M. A. (1994) Nucleic Acids Res. 22, 4653–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, L., Ci, L., Zhang, X. & Rich, A. (1994) Biochemistry 33, 13540–13546. [DOI] [PubMed] [Google Scholar]
- 16.Kang, C., Berger, I., Lockshin, C., Ratliff, R., Moyzis, R. & Rich, A. (1994) Proc. Natl. Acad. Sci. USA 91, 11636–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, C., Berger, I., Lockshin, C., Ratliff, R., Moyzis, R. & Rich, A. (1995) Proc. Natl. Acad. Sci. USA 92, 3874–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger, I., Kang, C., Fredian, A., Ratliff, R., Moyzis, R. & Rich, A. (1995) Nat. Struct. Biol. 2, 416–425. [DOI] [PubMed] [Google Scholar]
- 19.Gehring, K., Leroy, J.-L. & Gueron, M. (1993) Nature 363, 561–564. [DOI] [PubMed] [Google Scholar]
- 20.Guéron, M. & Leroy, J.-L. (2000) Curr. Opin. Struct. Biol. 10, 326–331. [DOI] [PubMed] [Google Scholar]
- 21.Viswamitra, M. A. & Pandit, J. (1983) J. Biomol. Struct. Dyn. 1, 743–753. [DOI] [PubMed] [Google Scholar]
- 22.Shepard, W., Cruse, W. B. T., Fourme, R., delaFortelle, E. & Prange, T. (1998) Structure (London) 6, 849–861. [DOI] [PubMed] [Google Scholar]
- 23.Spackova, N., Berger, I. & Sponer, J. (2000) J. Am. Chem. Soc. 122, 7564–7572. [Google Scholar]
- 24.Quigley, G. J. & Rich, A. (1976) Science 194, 796–806. [DOI] [PubMed] [Google Scholar]
- 25.Han, G. W. (2001) Acta Crystallogr. D 57, 213–218. [DOI] [PubMed] [Google Scholar]
- 26.Han, G. W., Kopka, M. L., Cascio, D., Grzeskowiak, K. & Dickerson, R. E. (1997) J. Mol. Biol. 269, 811–826. [DOI] [PubMed] [Google Scholar]
- 27.Otwinowski, Z. & Minor, W. (1997) in Methods in Enzymology: Macromolecular Crystallography, Part A., eds. Carter, C. W., Jr., & Sweet, R. M. (Academic, New York, NY), Vol. 276, pp. 307–326. [Google Scholar]
- 28.Kissinger, C. R., Gehlhaar, D. K. & Fogel, D. B. (1999) Acta Crystallogr. D 55, 484–491. [DOI] [PubMed] [Google Scholar]
- 29.Brünger, A. T. (1992) x-plor, A System for X-Ray Crystallography and NMR (Yale Univ. Press, New Haven, CT), Version 3.1.
- 30.Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D. 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 31.Sudarsanakumar, C., Xiong, Y. & Sundaralingam, M. (2000) J. Mol. Biol. 299, 103–112. [DOI] [PubMed] [Google Scholar]
- 32.Adams, P. D., Pannu, N. S., Read, R. J. & Brünger, A. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5018–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavery, R. & Sklenar, H. (1988) J. Biomol. Struct. Dyn. 6, 63–91. [DOI] [PubMed] [Google Scholar]
- 34.Sarafianos, S. G., Das, K., Tantillo, C., Clark, Jr., A. D., Ding, J., Whitcomb, J. M., Boyer, P. L., Hughes, S. H. & Arnold, E. (2001) EMBO J. 20, 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickerson, R. E. (1992) Methods Enzymol. 211, 67–111. [DOI] [PubMed] [Google Scholar]
- 36.Dock-Bregeon, A. C., Chevrier, B., Podjarny, A., Johnson, J., de Bear, J. S., Gough, G. R., Gilham, P. T. & Moras, D. (1989) J. Mol. Biol. 209, 459–474. [DOI] [PubMed] [Google Scholar]
- 37.Smith, C. K., Brannigan, J. A. & Moore, M. H. (1996) J. Mol. Biol. 263, 237–258. [DOI] [PubMed] [Google Scholar]
- 38.Dickerson, R. E., Goodsell, D. S. & Kopka, M. L. (1996) J. Mol. Biol. 256, 108–125. [DOI] [PubMed] [Google Scholar]
- 39.Saenger, W. (1984) in Principles of Nucleic Acid Structure, ed. Cantor, C. R. (Springer, New York), pp. 350–367.
- 40.Sobell, H. M., Tsai, C. C., Jain, S. C. & Gilbert, S. G. (1977) J. Mol. Biol. 114, 333–365. [DOI] [PubMed] [Google Scholar]
- 41.Wang, A. H.-J., Nathans, J., van der Marel, G., van Boom J. H. & Rich, A. (1978) Nature 276, 471–474. [DOI] [PubMed] [Google Scholar]
- 42.Wang, A. H.-J., Ughetto, G., Quigley, G. L. & Rich, A. (1986) J. Biomol. Struct. Dyn. 4, 319–342. [DOI] [PubMed] [Google Scholar]
- 43.Wang, A. H.-J., Ughetto, G., Quigley, G. J. & Rich, A. (1987) Biochemistry 26, 1152–1163. [DOI] [PubMed] [Google Scholar]
- 44.Kamitori, S. & Takusagawa, F. (1992) J. Mol. Biol. 225, 445–456. [DOI] [PubMed] [Google Scholar]
- 45.Gasper, S. M., Armitage, B., Shui, X., Hu, G. G., Yu, C., Schuster, G. B. & Williams, L. D. (1998) J. Am. Chem. Soc. 120, 12402–12409. [Google Scholar]
- 46.Berman, H. M., Neidle, S. & Stodola, R. K. (1978) Proc. Natl. Acad. Sci. USA 75, 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerson, R. E. (1999) in Oxford Handbook of Nucleic Acid Structure, ed. Neidle, S. (Oxford Univ. Press, New York), pp. 145–191.
- 48.Liu, Y., Hart, P. J., Schlunegger, M. P. & Eisenberg, D. S. (1998) Proc. Natl. Acad. Sci. USA 95, 3437–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong, Y. & Sundaralingam, M. (2000) Nucleic Acids Res. 28, 2171–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conn, G., Brown, T. & Leonard, G. (1999) Nucleic Acids Res. 27, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong, Y. & Sundaralingam, M. (1998) Structure (London) 6, 1493–1501. [DOI] [PubMed] [Google Scholar]
- 52.Horton, N. C. & Finzel, B. C. (1996) J. Mol. Biol. 264, 521–533. [DOI] [PubMed] [Google Scholar]
- 53.Timsit, Y., Vilbois, E. & Moras, D. (1991) Nature 354, 167–170. [DOI] [PubMed] [Google Scholar]
- 54.Treco, D. & Arnheim, N. (1986) Mol. Cell. Biol. 6, 3934–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]