Abstract
As locally converted estrogen from testicular testosterone contributes to apparent androgen activity, the physiological significance of androgen receptor (AR) function in the beneficial effects of androgens on skeletal tissues has remained unclear. We show here that inactivation of AR in mice using a Cre-loxP system-mediated gene-targeting technique caused bone loss in males but not in females. Histomorphometric analyses of 8-week-old male AR knockout (ARKO) mice showed high bone turnover with increased bone resorption that resulted in reduced trabecular and cortical bone mass without affecting bone shape. Bone loss in orchidectomized male ARKO mice was only partially prevented by treatment with aromatizable testosterone. Analysis of primary osteoblasts and osteoclasts from ARKO mice revealed that AR function was required for the suppressive effects of androgens on osteoclastogenesis supporting activity of osteoblasts but not on osteoclasts. Furthermore, expression of the receptor activator of NF-κB ligand (RANKL) gene, which encodes a major osteoclastogenesis inducer, was found to be up-regulated in osteoblasts from AR-deficient mice. Our results indicate that AR function is indispensable for male-type bone formation and remodeling.
Steroid sex hormones are essential for normal skeletal development and maintenance of healthy bone remodeling during adult life (1, 2). Sex hormone status reflects bone mass, such that hormonal deficiency is well known to lead to progressive bone loss (3). The most striking example, osteoporosis in postmenopausal women, is a state of estrogen deficiency coupled with imbalanced bone remodeling, with higher bone resorption than bone formation, and can be rescued by estrogen replacement (4). Although androgens seem to exert beneficial effects in both males and females (3, 5), the physiological role of the androgen–androgen receptor (AR) system in skeletal tissues has not yet been well established. This is because estrogens are locally converted from serum androgens by aromatase present in bone and seem to contribute to the overt androgen effects on the skeleton (5, 6). This concept is supported by observations that male mice deficient in either aromatase or skeletal major estrogen receptor (ERα) suffer bone loss (6, 7). However, a number of studies have demonstrated that androgen treatment is protective against bone loss caused by the impaired estrogen signaling (3, 5), raising the possibility that AR-mediated androgen effects in bone remodeling are physiologically significant and independent of estrogen actions.
AR is a member of the nuclear steroid/thyroid hormone receptor gene superfamily and acts as a ligand-inducible transcription factor (8, 9). Most androgen effects are thought to be exerted through the transcriptional control of particular sets of target genes by AR. Upon hormone binding, AR is translocated from the cytosol into the nucleus and binds specific promoter elements. A number of coregulators and/or coregulator complexes are then recruited to AR, which then activates or represses the transcription of various target genes (10–13). Like the well described target tissues for androgens, bone tissues also express AR, being present in both osteoblasts and osteoclasts (2). Reflecting this AR expression, androgens have been shown to regulate the expression of several genes encoding growth factors and cytokines that control bone remodeling via both osteoblasts and osteoclasts (2). However, the role of AR in bone cell gene expression has not been well studied because of the lack of animals and cell lines deficient in AR expression.
To define the physiological functions of AR in target tissues, an AR-null mutant mouse line was generated by means of the Cre–loxP system, which was used to circumvent the problem of male infertility (14). The present study found that 8-week-old male AR knockout (ARKO; ARL–/Y) mice developed osteopenia and exhibited retarded growth curves, although normal bone shape was retained and overt phenotypes were indistinguishable from those of WT female littermates. Although high bone turnover, with more bone resorption than formation, was seen in 8-week-old ARL–/Y male mice, female ARKO (ARL–/L–) mice seemed normal with respect to bone mass and bone remodeling. These findings suggest that AR function was essential for male-type bone mass and bone remodeling. Furthermore, up-regulation of receptor activator of NF-κB ligand (RANKL) gene expression, along with enhanced potency to induce osteoclastogenesis in an in vitro coculture system, was observed for AR-deficient osteoblasts. Taken together, our results indicate that AR performs a suppressive function in osteoblasts during osteoclastogenesis that is indispensable for normal male-type bone formation and remodeling.
Materials and Methods
Animals. ARKO mice with the original C57BL6/CBA hybrid background were generated and maintained as reported (14). All mice were maintained according to the protocol approved by the Animal Care and Use Committee of the University of Tokyo.
Identification of AR Transcript by RT-PCR. Total mRNA was extracted from excised femora and tibiae by using an Isogen kit (Wako Pure Chemical, Osaka) and reverse transcribed by using XL reverse transcriptase (Takara Shuzo, Otsu, Japan) and an oligo(dT) primer (Takara Shuzo). After first-strand cDNA synthesis, 5% of the reaction mixture was amplified with r-TaqDNA polymerase (Takara Shuzo) by using specific primer pairs: 5′-CATGTAGGCCATGAGGTCCACCAC-3′ and 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ for G3PDH; 5′-TGGTAGCTGGTACTTCTAATGC-3′ and 5′-CATAAGGTCCGGAGTAGTTCTC-3′ for AR-1; and 5′-CAGAAGTATCTATGTGCCAG-3′ and 5′-ATCTTCTGGGATGGGTCCTCAG-3′ for AR-2. Up to 35 cycles of amplification were performed, with each cycle consisting of 96°C for 30 s, 55°C for 60 s, and 72°C for 60 s.
Analysis of Skeletal Morphology. Bone radiographs of excised femora and tibiae from 8-week-old WT and ARKO littermates were taken by using a soft x-ray apparatus (CMB-2; Softex, Tokyo). Three-dimensional computed tomography scanning was performed by using a composite x-ray analyzing system (NX-HCP; NS-ELEX, Tokyo). Bone mineral density (BMD) was measured by dual energy x-ray absorptiometry using a bone mineral analyzer (Piximus densitometer; Lunar, Madison, WI). Skeletal preparations for morphology were performed as described (15). Trabecular bone parameters were measured in an area 1.2 mm in length from 0.1 mm below the growth plate at the proximal metaphysis of tibiae, and cortex bone parameters were measured at the midshaft of femora.
Orchidectomy and Hormone Replacement. Male WT and ARKO littermates were orchidectomized or sham operated at 3 weeks of age and implanted s.c. with a slow-releasing pellet (10 mg/60 days) (Innovative Research of America) of placebo, testosterone (T), or 5α-dehydrotestosterone (DHT). BMD of femora was compared among eight experimental groups at 8 weeks of age.
Measurement of Serum Bone Metabolic Markers. Serum osteocalcin and urinary deoxypyridinoline were measured as bone formation and resorption markers in male WT and ARKO mice. Blood was sampled at time of death, and urine was collected for 24 h before death by using oil-sealed bottles in metabolic cages (Kurea, Tokyo). Concentration of deoxypyridinoline was corrected according to urinary creatinine concentration.
Statistical Analysis. Group means were compared by ANOVA, and the significance of differences were determined by post hoc testing with Bonferroni's method.
Ex Vivo Osteoblast Cultures. Osteoblast cultures were performed as described (15). For the cell proliferation assay, primary osteoblasts were inoculated at a density of 2 × 103 cells per well in a 96-multiwell plate for 2 days, and cell number was counted with cell-counting kit solution (Wako Pure Chemical) according to the manufacturer's protocol. For the measurement of alkaline phosphatase activity, primary osteoblasts were inoculated at a density of 5 × 104 cells per 24 multiwells and cultured in αMEM containing 10% FBS and 50 μg/ml ascorbic acid. After 14 days of culture, alkaline phosphatase activity in the cell lysate was measured by using a Wako ALP kit (Wako Pure Chemical).
Osteoclastogenesis in Coculture of Bone Marrow Cells and Osteoblasts. To visualize osteoclasts, staining for tartrate-resistant acid phosphatase (TRAP) activity is widely used (15). TRAP-positive multinucleated osteoclasts were generated from hemopoietic cells by coculturing with osteoblasts. As a source of hemopoietic cells, bone marrow cells (5 × 105 cells per well) and osteoblasts (5 × 104 cells per well) were isolated and placed in 0.5 ml per 24 multiwells of αMEM/10% FBS for 8 days in the presence of 10 nM 1α,25(OH)2D3 (16). To determine osteoclast survival, osteoclasts were isolated and cultured as described (15). The number of TRAP-positive and trypan blue-negative multinucleated cells were counted at 0, 12, 18, 24, and 48 h. Bone resorption activity was measured as described (15).
Osteoclastogenesis in Bone Marrow Cell Culture. Bone marrow cells were inoculated at a density of 5 × 105 cells per 24 multiwells and cultured in 1.0 ml per 24 multiwells of αMEM/10% FBS with 100 ng/ml macrophage colony-stimulating factor (M-CSF) for the first 48 h, after which 100 ng/ml M-CSF and 100 ng/ml RANKL were added and incubated for an additional 96 h. TRAP-positive multinucleated osteoclasts were then counted.
RT-PCR Analysis of RANKL Gene Expression. Semiquantitative RT-PCR was carried out on primary cultured osteoblasts for a number of osteoblastic factor genes (17). After osteoblasts were cultured to conf luency in αMEM containing 10% FBS, 1α,25(OH)2D3 (10 nM) was added with DHT (10 nM) or vehicle and cultured for 48 h. Total RNA extraction and reverse transcription was performed as described above. After first-strand cDNA synthesis, 5% of the reaction mixture was amplified with r-TaqDNA polymerase (Takara Shuzo) by using specific primer pairs 5′-GCATCGCTCTGTTCCTGTA-3′ and 5′-GTGCTCCCTCCTTTCATCA-3′ for RANKL and G3PDH. Up to 25 cycles of amplification were performed, with each cycle consisting of 96°C for 30 s, 55°C for 60 s, and 72°C for 60 s.
Results
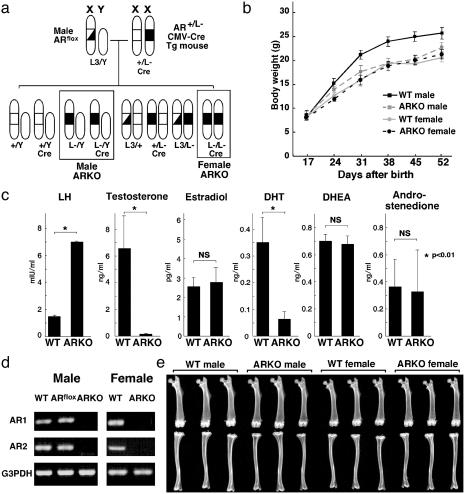
Male ARKO Mice Exhibit Abnormalities Typical of Testicular Feminization Mutant Rodents. To avoid male infertility caused by AR mutation, thereby preventing the establishment of a line of AR-deficient mice, we applied the Cre-loxP system to first flox the AR gene on the sole X chromosome in male mice (14). Floxed male mice (ARL3/Y) (Fig. 1a) seemed completely normal with regard to bone mass and reproduction and showed no apparent differences from WT littermates. Male ARKO (ARL–/Y) mice, descended from female AR heterozygotes (AR+/L–), showed growth retardation compared with male littermates, but growth curves and external reproductive organs were indistinguishable from those of female littermates up to 8 weeks of age (Fig. 1b). Reflecting the atrophic testis in 8-week-old ARL–/Y mice, testicular androgen production seemed to be severely impaired, leading to marked reduction in serum gonadal androgen levels, whereas serum estrogen and adrenal androgen levels remained unchanged (Fig. 1c). Thus, the phenotypic abnormality of ARL–/Y mice seemed identical to that reported for testicular feminization mutant rodents, natural mutants with AR dysfunction (3, 10). To our surprise, female ARKO (ARL–/L–), which never exist in nature, exhibited no phenotypic abnormality up to 8 weeks of age. However, it was possible that the abnormal AR protein derived from the genetically mutated AR gene locus still retained some level of androgen responsiveness (18). To address this issue, the presence of AR transcripts in bone in both male (ARL–/Y) and female (ARL–/L–) ARKO mice was assessed. No transcripts were detected by Northern blotting (data not shown) or by RT-PCR using several primer pairs performed on total bone tissue (Fig. 1d). These findings established that our ARKO mouse was indeed a mammalian AR-null mutant model.
Fig. 1.
(a) Strategy to generate male and female ARKO mice using CMV-Cre transgenic mice. (b) Growth curves of ARKO and WT littermate mice. (c) Serum hormone levels in 8-week-old ARKO and WT mice. (d) Lack of AR expression in bone of ARKO mice (RT-PCR). (e) Soft x-ray images of femora and tibiae from 8-week-old ARKO and WT mice.
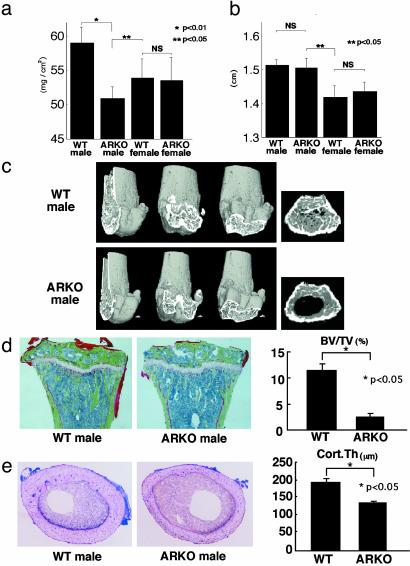
Osteopenia in Male ARKO Mice. To examine the physiological roles of AR in mediating androgenic effects on bone tissue, we first analyzed the skeletal morphology of ARL–/Y mice. Soft x-ray analysis revealed severe osteopenia of femora and tibiae from 8-week-old male mice (Fig. 1e), but also that bone shape and bone length (Figs. 1e and 2b) were not affected by AR inactivation. This was consistent with the clear loss in radiographic BMD of femora from ARL–/Y mice compared with WT male littermates. The BMD of ARL–/Y mice was even lower than that of WT female littermates (Fig. 2a). Three-dimensional computed tomography scan analysis of femora showed that bone loss in ARL–/Y mice occurred throughout the entire femur (Fig. 2c). Histomorphometric analysis of proximal tibiae from ARL–/Y mice confirmed that trabecular bone volumes (bone volume per tissue volume: BV/TV) were markedly decreased, whereas no overt differences in growth plates were detected between ARL–/Y and WT mice (Fig. 2d). Such bone loss was also evident in cortical bones as estimated by cortex thickness (Fig. 2e). In contrast, no clear loss of bone mass was detected in female ARKO mice (Figs. 1e and 2a). Together, these findings suggest that ARL–/Y mice developed osteopenia, which implies that the AR function is required for male-type bone mass and remodeling.
Fig. 2.
Osteopenia in male ARKO mice. (a) Bone loss in femur of 8-week-old male ARKO mice by BMD analysis. (b) Bone length in 8-week-old ARKO and WT littermate mice. (c) Three-dimensional computed tomography images of distal femora and axial sections of distal metaphyses from representative 8-week-old male WT and ARKO littermates. (d) Histological features and histomorphometry of the proximal tibiae from 8-week-old male ARKO and WT mice. For Villanueva–Goldner staining of sections from representative ARKO and WT male littermates, mineralized bone is stained green. BV/TV, trabecular bone volume expressed as a percentage of total tissue volume. (e) Histological features and histomorphometry of the midshaft of femora of 8-week-old mice. Cort.Th., cortex thickness.
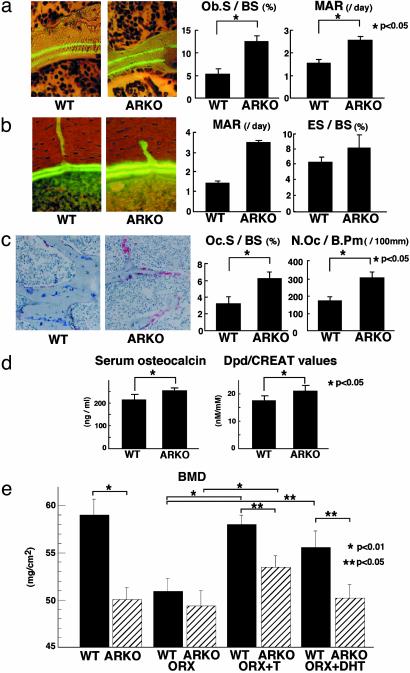
The Suppressive Function of AR in Bone Resorption Is Abrogated in Male ARKO Mice. To further study the process of bone loss in ARL–/Y mice, bone remodeling was assessed. The bone formation rate of the trabecular bone was directly estimated by calcein double-labeling of the mineralized matrix (17). Unexpectedly, the bone formation parameters (Ob.S/BS and MAR) were significantly increased in ARL–/Y mice, with increased thickness of the region between the two calcein-labeled layers compared with WT littermates (Fig. 3a). This increased bone formation was also found in the cortical bone, in the endocortical area of the axial section (Fig. 3b). We observed an increase in the number of TRAP-positive mature osteoclasts (Fig. 3c). This was reflected in the significantly increased bone resorption parameters (Oc.S/BS, N.Oc/B.Pm) (Fig. 3c). Our finding of enhanced bone turnover was further supported by the increased levels of serum osteocalcin and urinary deoxypyridinoline (Fig. 3d). Although female ARKO mice exhibited no abnormal bone remodeling, our findings suggested that AR inactivation caused high turnover bone remodeling with higher bone resorption than formation, resulting in the development of osteopenia in males.
Fig. 3.
High bone turnover in male ARKO mice and its partial prevention by an aromatizable androgen. (a) Two calcein-labeled mineralized fronts visualized by fluorescent micrography and bone formation parameters in the proximal tibia of 8-week-old male ARKO and WT littermate mice. Ob.S/BS, percentage of bone surface covered by cuboidal osteoblasts; MAR, mineral apposition rate. (b) Two calcein-labeled mineralized fronts and bone histomorphometric parameters in the midshaft of femora from 8-week-old male ARKO and WT littermate mice. ES/BS, percentage of eroded surface. (c) TRAP staining and bone resorption parameters in the proximal tibiae of 8-week-old male WT and ARKO mice. TRAP-positive osteoclasts on secondary spongiosa are stained red. Oc.S/BS, percentage of bone surface covered by mature osteoclasts; N.Oc/B.Pm, number of mature osteoclasts in 10 cm of bone perimeter. (d) Serum osteocalcin levels and urinary deoxypyridinoline levels of 8-week-old male ARKO and WT mice. (e) BMD values of femora from 8-week-old mice after orchidectomy (ORX) and hormone replacement. T, testosterone.
Effects of Androgens Other Than Those from Locally Converted Estrogen on Male Bone Remodeling. It is thought that estrogen converted from serum testosterone by aromatase expressed in androgen target tissues, including bone, contributes to apparent androgen activity (6). As serum testosterone levels were drastically decreased due to atrophic testes in ARL–/Y mice, it was impossible to exclude the possibility that the osteopenia in ARL–/Y mice simply reflected the impaired action of converted estrogen from serum testosterone on skeletal tissues. To address this issue, aromatizable testosterone (T) and nonaromatizable androgen DHT were given to orchidectomized ARL–/Y and WT littermates, and the femur BMD was assessed. Whereas orchidectomy caused no further BMD loss in ARL–/Y femora, BMD values from orchidectomized WT littermates were higher, but not significantly, than those of ARL–/Y mice (Fig. 3e). Also, whereas T, but not DHT, effectively recovered BMD, the recovery in ARL–/Y mice due to T was only ≈50% of that in intact males (Fig. 3e), which suggests that androgen actions mediated via AR are significant in protecting against bone loss.
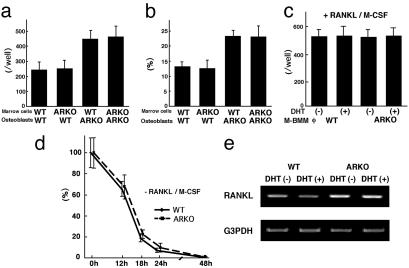
Suppressive Effects of AR on in Vitro Osteoclastogenesis and RANKL Gene Expression in Osteoblasts. To further explore the mechanisms of increased bone resorption in ARL–/Y mice, in vitro osteoclastogenesis was assessed using osteoclast precursor cells from bone marrow and calvaria osteoblasts (16, 17) derived from male WT and ARL–/Y mice (Fig. 4a). TRAP-positive multinucleated osteoclasts were clearly cytodifferentiated by 1α,25(OH)2D3 stimulation even when AR was absent in osteoclast precursor cells (Fig. 4a). RANKL is a member of tumor necrosis factor-α superfamily cytokines, which induces osteoclastogenesis and activates osteoclast function. Consistent with normal osteoclastogenesis in response to 1α,25(OH)2D3, RANKL signaling activated by RANKL and M-CSF during osteoclastogenesis also seemed unaffected in AR-deficient osteoclasts (Fig. 4c). Moreover, resorption pits produced by cultured AR-deficient primary osteoclasts seemed normal both in terms of numbers and area when compared with osteoclasts from WT littermates (Fig. 4b). Surprisingly, the survival rate of osteoclasts in the presence of DHT was unaffected by AR inactivation (Fig. 4d), in sharp contrast to a recent report concerning the osteoclast survival function of AR (19). However, AR inactivation in osteoblasts seemed to potentiate osteoblastic functions that promote osteoclastogenesis in the presence of inducers (Fig. 4 a and b). However, we detected no abnormalities in cultured AR-deficient primar y osteoblast cell proliferation by MT T [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay or maturation by alkaline phosphatase activity (data not shown). In searching for the osteoblastic factor responsible for the increased osteoclastogenesis (20, 21), RANKL turned out to be transcriptionally up-regulated in AR-deficient osteoblasts (Fig. 4e). Thus, our findings suggest that the suppressive function of AR on RANKL gene expression mediates the protective effects of androgen on bone remodeling through the inhibition of bone resorption.
Fig. 4.
(a) Osteoclastogenesis in bone marrow cell and osteoblast cocultures. TRAP-positive multinucleated osteoclast numbers were counted after 8-day coculture of bone marrow cells and osteoblasts from male ARKO and WT mice in the presence of 10 nM 1α,25(OH)2D3. (b) Pit area resorbed by osteoclasts over an additional 48-h period of coculture on a dentine slice. (c) Osteoclast formation in cultured M-CSF-dependent bone marrow macrophages. (d) Survival rate of isolated osteoclasts formed during coculture of osteoblasts and bone marrow cells. (e) Gene expression of RANKL and G3PDH in cultured primary osteoblasts from male ARKO and WT littermates.
Discussion
The present study, using orchidectomized ARKO mice, directly demonstrates that AR function is essential for androgen effects on the male skeleton. Although a number of previous studies have assessed the bone phenotypes of testicular feminization mutant rodents and humans eventually treated with sex hormones (3, 5), it has still been difficult to isolate AR functions in the skeletal system due to the local conversion of androgens to estrogens (6). When aromatizable testosterone (Fig. 3e) was given to orchidectomized ARKO mice, bone loss was only partially prevented, clearly establishing the pivotal function of AR in male-type bone remodeling. This finding is supported by previous reports concerning the beneficial effects of testosterone in male ERα–/– and ERαβ–/– bone (22). As no clear bone loss in female ARKO mice was detected, AR is likely to be one of the determining factors in the formation of male-type bone, along with other AR downstream factors that may be encoded in male-specific Y chromosome regions. However, as bone loss was also induced by inactivation of either ERα or aromatase in male mice (23, 24), ER-mediated estrogen signaling also seems to be physiologically significant in the retention of male-type bone mass and bone remodeling. In such mice, high bone turnover with increased bone resorption was also observed. Therefore, it is possible that receptor-mediated signaling by both androgens and estrogens is convergent in the suppressive action on male bone resorption.
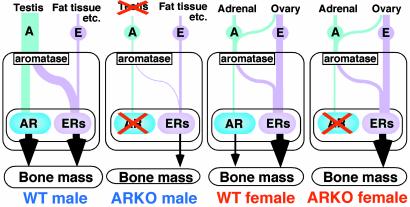
Unlike male ARKO mice, no overt differences in bone phenotype and mass were found between female AR-deficient and WT littermate mice at 8 weeks of age. Although the effects of anabolic androgens on bone have been documented in female rodents and humans, the physiological function of AR may not be significant because of the probably dominant role of ER function in female bones (Fig. 5), reflecting the high levels of serum estrogens in females. However, under certain conditions, such as estrogen deficiency in postmenopausal women (4), AR-mediated androgen effects may become physiologically significant in the protection against bone loss. This hypothesis will be tested using ovariectomized female ARKO mice in future studies.
Fig. 5.
Schema of skeletal sex hormone action. In male WT mice, skeletal sex hormone activities are mediated by both AR and ER. In female WT mice, skeletal function of ER is likely to dominate over that of AR as serum levels of AR ligands in females are quite low. In male ARKO mice, testicular testosterone production is severely impaired by hypoplasia of the testes, leading to a lack of skeletal sex hormone actions. In contrast, female ARKO mice may not be greatly affected by disruption of AR signaling.
The higher rate of bone resorption than formation with increased osteoclastogenesis observed in the ARL–/Y mice has been implied in previous reports (3, 5, 25). However, several recent reports (19, 26, 27) were inconsistent with our findings that osteoclast precursor cells were unaffected by AR deficiency in terms of cell survival and responsiveness to well characterized osteoclastogenesis inducers such as 1α,25(OH)2D3 and M-CSF (Fig. 4 a–d). Similar to AR, critical ERα functions in osteoclast precursor cells from ERα-deficient (ERα–/–) mice were not detected during in vitro osteoclastogenesis, and increased expression of the RANKL gene in osteoblast was also found (unpublished results). Together with previous findings of suppressive ERα effects on RANKL signaling (28), we speculate that the convergent functions of AR and ERα in male bone formation and remodeling are mediated through their suppressive functions on RANKL gene expression in osteoblasts. To test this hypothesis, identification of the negatively regulatory elements by receptors in the RANKL gene promoter (29) is clearly required.
Acknowledgments
We thank Dr. Yoshiaki Azuma and the hard tissue research team at Kureha Chemical for technical assistance and Dr. Toru Akune for helpful discussion. This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (to S.K. for priority areas and to H. Kawano).
Abbreviations: AR, androgen receptor; ARKO, AR knockout; ER, estrogen receptor; BMD, bone mineral density; DHT, 5α-dehydrotestosterone; RANKL, receptor activator of NF-κB ligand; TRAP, tartrate-resistant acid phosphatase; M-CSF, macrophage colony-stimulating factor.
References
- 1.Bland, R. (2000) Clin. Sci. 98, 217–240. [PubMed] [Google Scholar]
- 2.Compston, J. E. (2001) Physiol. Rev. 81, 419–447. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian, J. P. (2002) J. Bone Miner. Res. 17, 563–566. [DOI] [PubMed] [Google Scholar]
- 4.Riggs, B. L., Khosla, S. & Melton, L. J., III (2002) Endocr. Rev. 23, 279–302. [DOI] [PubMed] [Google Scholar]
- 5.Hofbauer, L. C. & Khosla, S. (1999) Eur. J. Endocrinol. 140, 271–286. [DOI] [PubMed] [Google Scholar]
- 6.Simpson, E. R. & Davis, S. R. (2000) Proc. Natl. Acad. Sci. USA 97, 14038–14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couse, J. F. & Korach, K. S. (1999) Endocr. Rev. 20, 358–417. [DOI] [PubMed] [Google Scholar]
- 8.Takeyama, K., Ito, S., Yamamoto, A., Tanimoto, H., Furutani, T., Kanuka, H., Miura, M., Tabata, T. & Kato, S. (2002) Neuron 35, 855–864. [DOI] [PubMed] [Google Scholar]
- 9.Mangelsdorf, D. J., Thummel, C., Beato, M., Herrlich, P., Schutz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P., et al. (1995) Cell 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson, J. D. (1999) Endocr. Rev. 20, 726–737. [DOI] [PubMed] [Google Scholar]
- 11.McKenna, N. J. & O'Malley, B. W. (2002) Cell 108, 465–474. [DOI] [PubMed] [Google Scholar]
- 12.Glass, C. K. & Rosenfeld, M. G. (2000) Genes Dev. 14, 121–141. [PubMed] [Google Scholar]
- 13.Yanagisawa, J., Kitagawa, H., Yanagida, M., Wada, O., Ogawa, S., Nakagomi, M., Oishi, H., Yamamoto, Y., Nagasawa, H., McMahon, S. B., et al. (2002) Mol. Cell 9, 553–562. [DOI] [PubMed] [Google Scholar]
- 14.Sato, T., Matsumoto, T., Yamada, T., Watanabe, T., Kawano, H. & Kato, S. (2003) Biochem. Biophys. Res. Commun. 300, 167–171. [DOI] [PubMed] [Google Scholar]
- 15.Ogata, N., Chikazu, D., Kubota, N., Terauchi, Y., Tobe, K., Azuma, Y., Ohta, T., Kadowaki, T., Nakamura, K. & Kawaguchi, H. (2000) J. Clin. Invest. 105, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda, S., Yoshizawa, T., Nagai, Y., Yamato, H., Fukumoto, S., Sekine, K., Kato, S., Matsumoto, T. & Fujita, T. (1999) Endocrinology 140, 1005–1008. [DOI] [PubMed] [Google Scholar]
- 17.Nakamichi, Y., Shukunami, C., Yamada, T., Aihara, K., Kawano, H., Sato, T., Nishizaki, Y., Yamamoto, Y., Shindo, M., Yoshimura, K., et al. (2003) Mol. Cell. Biol. 23, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspar, M. L., Meo, T., Bourgarel, P., Guenet, J. L. & Tosi, M. (1991) Proc. Natl. Acad. Sci. USA 88, 8606–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kousteni, S., Chen, J. R., Bellido, T., Han, L., Ali, A. A., O'Brien, C. A., Plotkin, L., Fu, Q., Mancino, A. T., Wen, Y., et al. (2002) Science 298, 843–846. [DOI] [PubMed] [Google Scholar]
- 20.Teitelbaum, S. L. (2000) Science 289, 1504–1508. [DOI] [PubMed] [Google Scholar]
- 21.Ducy, P., Schinke, T. & Karsenty, G. (2000) Science 289, 1501–1504. [DOI] [PubMed] [Google Scholar]
- 22.Sims, N. A., Dupont, S., Krust, A., Clement-Lacroix, P., Minet, D., Resche-Rigon, M., Gaillard-Kelly, M. & Baron, R. (2002) Bone 30, 18–25. [DOI] [PubMed] [Google Scholar]
- 23.Vidal, O., Lindberg, M. K., Hollberg, K., Baylink, D. J., Andersson, G., Lubahn, D. B., Mohan, S., Gustafsson, J. A. & Ohlsson, C. (2000) Proc. Natl. Acad. Sci. USA 97, 5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyaura, C., Toda, K., Inada, M., Ohshiba, T., Matsumoto, C., Okada, T., Ito, M., Shizuta, Y. & Ito, A. (2001) Biochem. Biophys. Res. Commun. 280, 1062–1068. [DOI] [PubMed] [Google Scholar]
- 25.Yeh, S., Tsai, M. Y., Xu, Q., Mu, X. M., Lardy, H., Huang, K. E., Lin, H., Yeh, S. D., Altuwaijri, S., Zhou, X., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevde, N. K., Bendixen, A. C., Dienger, K. M. & Pike, J. W. (2000) Proc. Natl. Acad. Sci. USA 97, 7829–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber, D. M., Bendixen, A. C., Pathrose, P., Srivastava, S., Dienger, K. M., Shevde, N. K. & Pike, J. W. (2001) Endocrinology 142, 3800–3808. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg, M. K., Erlandsson, M., Alatalo, S. L., Windahl, S., Andersson, G., Halleen, J. M., Carlsten, H., Gustafsson, J. A. & Ohlsson, C. (2001) J. Endocrinol. 171, 425–433. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa, R., Kitazawa, S. & Maeda, S. (1999) Biochim. Biophys. Acta 1445, 134–141. [DOI] [PubMed] [Google Scholar]