Abstract
The mechanisms by which the hereditary hemochromatosis protein, HFE, decreases transferrin-mediated iron uptake were examined. Coimmunoprecipitation studies using solubilized cell extracts demonstrated that transferrin (Tf) competed with HFE for binding to the transferrin receptor (TfR) similar to previous in vitro studies using soluble truncated forms of HFE and the TfR. At concentrations of Tf approaching those found in the blood, no differences in Tf binding to cells were detected, which is consistent with the lower binding constant of HFE for TfR versus Tf. However, cells expressing HFE still showed a decrease in Tf-mediated iron uptake at concentrations of Tf sufficient to dissociate HFE from the TfR. These results indicate that the association of HFE with TfR is not essential for its ability to lower intracellular iron stores. To test the effect of HFE on lowering intracellular iron levels independently of its association with TfR, a mutated HFE (fW81AHFE) that shows greatly reduced affinity for the TfR was transfected into tetracycline-controlled transactivator HeLa cells. HeLa cells expressing fW81AHFE behaved in a similar manner to cells expressing wild-type HFE with respect to decreased intracellular iron levels measured by iron regulatory protein gel-shift assays and ferritin levels. The results indicate that HFE can lower intracellular iron levels independently of its interaction with the TfR.
Type 1 hereditary hemochromatosis (HH) is an autosomal recessive disorder with the clinical manifestation of severe iron overload in specific organs, including liver, heart, and pancreas. It is the most common inherited disease in people of northern European descent, affecting ≈1 in 400 individuals (reviewed in refs. 1 and 2). The HH gene encodes an atypical MHC class I-related protein named HFE. About 85% of Type 1 HH patients are homozygous for a single base pair mutation that results in a substitution of tyrosine for cysteine at amino acid position 260 (C260Y) (3) [the numbering system for the amino acids in HFE reflects the amino acid position in the mature protein]. The C260Y mutation of HFE prevents the formation of a disulfide bond in its α3 domain and abrogates its association with β2-microglobulin (β2m) as well as its subsequent trafficking to the cell surface (4, 5). The importance of functional HFE–β2m heterodimers for iron homeostasis is supported by the studies using gene-targeted animals. HFE (-/-) (6), β2m (-/-) (7, 8), and β2m (-/-)-HFE (-/-) double knockout mice (9) show similar patterns and extents of iron overload as individuals with HH.
Although HFE is expressed in most tissues (3), regulation of iron absorption in the intestine by HFE has been presumed to be the critical step to maintain the iron homeostasis (reviewed in ref. 1). In HFE(-/-) knockout mice, intestinal iron absorption by divalent metal transporter 1 is enhanced (10) and duodenal uptake of plasma transferrin (Tf) is impaired, but iron uptake by the kidney and liver is normal (11).
How HFE regulates iron absorption at the molecular level still remains elusive. In vivo and in vitro studies show that HFE forms a complex with the Tf receptor (TfR) at neutral pH (12–14). The binding sites on TfR for HFE and Tf overlap (15), which explains the observation that HFE is able to lower the apparent binding affinity of TfR to diferric Tf (13, 16). These results predict that, at circulating concentrations of diferric Tf (≈10 μM), no HFE should be binding to the TfR. Indeed, previous studies indicate that even at 100 nM to 1 μM Tf, no difference in Tf uptake was measured in cells expressing HFE compared with cells not expressing HFE (17). Despite this, cells expressing HFE have 30% less iron accumulation (17).
In this study we used a mutated HFE (W81A), which has a 5,000-fold lower affinity for the TfR than the WT HFE, to determine the effect of the HFE/TfR complex on Tf-mediated iron uptake. tTA HeLa cells expressing an epitope-tagged WT (fWT)-, fW81A-, or fC260Y-mutated HFE under the tight control of a tetracycline-repressible promoter were used. HFE expression was repressed in the presence of doxycycline (Dox, a tetracycline analog). Surprisingly, we found that the decrease in Tf-iron uptake upon HFE expression is independent of HFE binding to the TfR.
Experimental Methods
Cell Line Preparations and Western Analysis. Both W81A and C260Y mutations of human HFE were made by using QuikChange XL Site-Directed Mutagenesis kit (Strategene) following the manufacturer's instructions. The previously made fWTHFE/pUHD10-3 construct with a C-terminal Flag tag (f) was used as a template (16). The primers used for the W81A mutation are 5′-GGG ATC ACA TGT TCA CTG TTG ACT TCG CGA CTA TTA TGG-3′ (forward) and 5′-CCA TAA TAG TCG CGA AGT CAA CAG TGA ACA TGT GAT CCC-3′ (reverse). The primers used for the C260Y mutation are 5′-GCA GAG ATA TAC GTA CCA GGT GGA GCA CC-3′ (forward) and 5′-GGT GCT CCA CCT GGT ACG TAT ATC TCT GC-3′ (reverse). The W81A and C260Y mutations in the resulting constructs were confirmed by DNA sequencing. No other new sequence changes were detected. Cells were transfected as described (16) and maintained in DMEM supplemented with 10% FBS, 400 μg/ml G418 (Geneticin, Calbiochem), and 300 ng/ml puromycin with or without 1 μg/ml Dox.
The expression of HFE in both fW81AHFE/tTA and fC260YHFE/tTA HeLa cells was found tightly controlled by Dox by Western blot analysis using rabbit anti-HFE antibody (1:10,000 dilution, 137, J. Feder, Bristol-Meyers Squibb). These cell lines and our previously developed cell line fWTHFE/tTA HeLa (16) were all tested for the levels of β2m and ferritin (Ft) after turning off the HFE expression for different time intervals by adding Dox. The antibodies used were mouse anti-β2m antibody (1:1,000 dilution) (Immunotech, Luminy, France), and rabbit anti-Ft antibody (1:500 dilution, DAKO). The appropriate secondary antibodies conjugated to horseradish peroxidase were applied, and the chemiluminescence kit (Super Signal, Pierce) was adopted to develop according to the manufacturer's directions. For all of the above analysis, 30 μg of protein from whole cell lysates were subjected to SDS/12% PAGE and transferred to nitrocellulose.
Immunoprecipitation. Immunoprecipitation was performed as described (16). Mouse monoclonal antibodies, M2 to the Flag epitope (Sigma) and 8C10 (gift of R. Ehrlich, Tel Aviv University, Tel Aviv), were used for immunoprecipitation of HFE. A mouse monoclonal anti-human TfR antibody 4091 (gift of V. Landt, Washington University, St. Louis) was used for TfR.
Immunofluorescence. Immunofluorescent analysis of HFE and TfR localization in both fW81AHFE/tTA and fWTHFE/tTA HeLa (Dox- and Dox+) cells was processed as described (16). Mouse anti-HFE (8C10, 1:1,000 dilution in blocking buffer) or sheep anti-TfR (1:800 dilution) antibody was used as the primary, and Alexa 594 donkey anti-mouse (1:250 dilution) or Alexa 488 donkey anti-sheep (1:500 dilution, Molecular Probes) was used as the secondary. Cells were mounted with ProLong Antifade (Molecular Probes) and imaged by the Oregon Health Sciences University/Medical Marketing International Research Core Facility with the Applied Precision Deltavision image restoration system on a Nikon microscope (×60 oil immersion lens).
125I-Tf Binding and Tf-55Fe Uptake. Human holo-Tf was labeled with Na125I by using lactoperoxidase as described (18, 19). 125I-Tf binding assays of fW81AHFE/tTA and fWTHFE/tTA HeLa cells under both Dox+ and Dox- conditions were performed as described (18, 19). All of the samples were assayed in triplicate. Results were expressed as fmol 125I-Tf per 106 cells. 55Fe loading onto human apo-Tf and the Tf-55Fe uptake were the same as described (17).
Electrophoretic Mobility-Shift Assay. Iron regulatory protein (IRP) binding to Ft iron-responsive element (IRE) mRNA was performed and quantitated as described (20).
Results
A series of experiments were done to reconcile the fact that, at concentrations of Tf that can effectively compete with HFE for binding to TfR, less iron accumulates in cells expressing HFE. The rates of Tf-55Fe uptake and 125I-Tf binding at various concentrations of human diferric Tf were examined to compare the effect of HFE on Tf binding and Tf-mediated iron uptake. One way to account for these results would be if HFE has to be released from TfR by Tf to lower transferrin-mediated iron uptake into cells. A mutated HFE (fW81AHFE), which has greatly reduced affinity for TfR but is still able to bind to β2m and be transported to the cell surface, was stably transfected into cells to test this possibility.
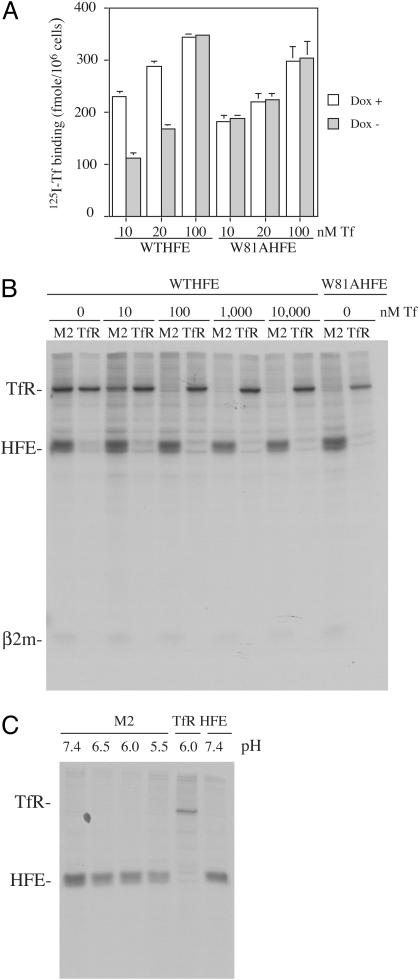
HFE Decreases Tf-Mediated Iron Uptake Independent of Its Competition with Tf for Binding to the TfR. The W81AHFE mutation was originally designed to measure the contribution of specific amino acids to the HFE-TfR interface (21). This previous study showed that the affinity of HFE for TfR decreased ≈5,000-fold with the W81A mutation. The W81AHFE is still able to bind β2m and form a heterodimer. The C260YHFE mutation, the most common mutation associated with HH, was generated as a control. This mutation disrupts HFE's association with β2m and TfR and does not alter the iron status of cells (12, 13, 22). W81A and C260Y mutations in HFE with a Flag epitope tag were generated and stably transfected into the tTA HeLa cell line. The expression of HFE was repressed in the presence of Dox (Dox+). The binding of 125I-Tf to fW81AHFE and fWTHFE cells at 10, 20, and 100 nM Tf was measured. No inhibition of Tf binding to fW81AHFE cells or the control fC260YHFE cells (data not shown) was detected at any concentration of Tf (Fig. 1A). In contrast, fWTHFE cells showed decreased 125I-Tf binding at subsaturating concentrations of Tf. In agreement with plasmon resonance binding studies using truncated forms of the TfR and HFE, the lowered binding of Tf to cells expressing HFE at low concentrations but not at high concentrations of Tf is consistent with HFE and Tf competing for overlapping binding sites on TfR.
Fig. 1.
Evidence for the competition between Tf and HFE for binding to the TfR in intact cells and cell lysates. (A) Binding of 125I-Tf to cells. fWTHFE/tTA and fW81AHFE/tTA HeLa cells expressing HFE (Dox-) or not (Dox+) were incubated with 10, 20, and 100 nM 125I-Tf for 90 min at 4°C to allow binding to cell surface TfRs. Excess unlabeled diferric Tf (200×) was added to measure nonspecific binding. Samples were done in triplicate. (B) Coimmunoprecipitation of HFE in the presence of Tf. fWTHFE/tTA HeLa cells expressing HFE (Dox-) were labeled overnight with 50 μCi/ml [35S]methionine/cysteine (1 Ci = 37 GBq). Cell lysates were preabsorbed with protein A beads, and diferric Tf was added for 30 min at 4°C, followed by immunoprecipitation with an anti-Flag antibody (M2) for HFE or a mouse monoclonal TfR antibody. The experiment was repeated twice with similar results. (C) Coimmunoprecipitation of fW81AHFE at pH 7.4, 6.5, 6.0, and 5.5. fW81AHFE/tTA cells (Dox-) were labeled with [35S]methionine/cysteine as described above. For each sample, pH was kept the same throughout the entire immunoprecipitation and washes. The same antibodies were used as above except in the last lane, where a mouse monoclonal (8C10) against HFE was used as a control.
Lower Tf binding by the fW81AHFE/tTA HeLa cells compared with fWTHFE/tTA HeLa cells under Dox repression of HFE expression was measured (Fig. 1 A). We attribute the lower binding to clonal variation and not to experimental variation or leakiness of the cells. This result was repeatable and did not appear to be caused by the inability to turn off expression because, as with the fWTHFE-expressing cells, no HFE could be detected by Western analysis when cells are incubated in the presence of 1 μg/ml Dox.
Coimmunoprecipitation was used to examine whether Tf is able to displace HFE from the TfR/HFE complex. Cell lysates from [35S]methionine-cysteine labeled cells were incubated with different concentrations of diferric Tf (0–10 μM) for 30 min at 4°C, followed by immunoprecipitation of HFE or TfR. A significant amount of HFE dissociated from TfR at the concentration of diferric Tf as low as 10 nM (Fig. 1B). The total amounts of TfR and HFE were unchanged. The same amount of TfR was immunoprecipitated by the anti-Flag antibody as by the anti-TfR antibody, which indicated a relatively stable TfR/HFE complex was formed and all of the TfR was associated with HFE. However, the reverse was not true. The monoclonal antibody to TfR appeared to compete with the binding of HFE to TfR and little HFE was isolated with TfR. The addition of Tf to the extracts followed by immunoprecipitation with anti-Flag antibody and the 125I-Tf binding studies indicated that Tf was able to displace HFE from the TfR in the context of the cell and cell lysates. The concentrations of Tf needed to displace HFE from the TfR were consistent with those of the competition studies between the ectodomain of HFE and Tf for binding to the ectodomain of TfR as measured by surface plasmon resonance (12, 15).
fW81AHFE could interact with TfR in the endosome. Isolation of fW81AHFE at pH 5.5–7.4 failed to detect TfR in the immunoprecipitates (Fig. 1C). These results are not surprising in that an amino acid substitution of one neutral amino acid for another would not be expected to impart a pH-sensitive change in binding. The fact that Tf remains associated with TfR at endosomal pH and that no interaction between fW81AHFE and TfR was detected at low pH rules out the possibility that fW81AHFE exerts its effect on Tf-mediated iron uptake into cells through interaction with the TfR in the endosome.
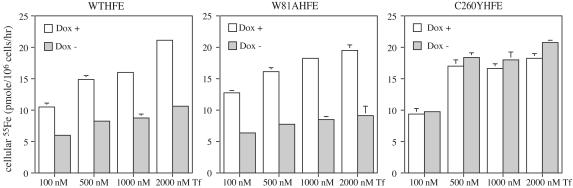
The amount of 55Fe taken up into cells via Tf at concentrations of diferric Tf that saturate binding to the TfR (100 nM to 2 μM) was examined to determine whether the fW81AHFE cells showed altered Tf-mediated iron uptake. They exhibited a similar extent of inhibition (≈40% decrease) on Tf-meditated iron uptake compared with cells expressing fWTHFE at all of the examined Tf concentrations (0.1, 0.5, 1, and 2 μM) (Fig. 2). These results suggest that HFE can function to lower iron levels in cells even in the absence of its association with TfR.
Fig. 2.
Amount of Tf-mediated 55Fe uptake at different concentrations of diferric Tf by fWTHFE/tTA, fW81AHFE/tTA, and fC260YHFE/tTA HeLa cells. Tf-55Fe was incubated with cells for 1 h at 37°C at 5% CO2 in medium without FCS. For each cell line, cells were expressing (Dox-) or not expressing (Dox+) HFE. The experiment was repeated twice with similar results.
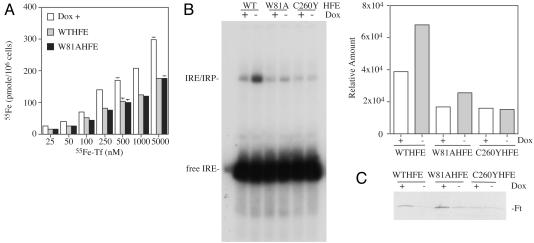
fWTHFE- and fW81AHFE-Expressing Cells Show Decreased Intracellular Iron Levels. We used three different methods to determine whether the cells expressing the different forms of HFE became iron deficient when HFE expression was induced. 55Fe-Tf uptake over longer periods was measured to determine steady state levels of Tf-delivered iron in cells. Cells were grown for 20 h in complete medium supplemented with 25 nM to 5 μM 55Fe-Tf, followed by acid wash to remove the membrane-associated 55Fe-Tf. Consistent with the short-term uptake studies, cells expressing both the fWTHFE and fW81AHFE had lower accumulation of 55Fe (Fig. 3A). Gel-shift analysis of IRPs was used to further study the steady state levels of intracellular iron. At low intracellular iron concentrations, IRPs bind to the stem loop structure of the IRE in the untranslated region of mRNA, whereas at high intracellular iron concentrations the iron–sulfur complex in IRP1 and ubiquitin-mediated degradation of IRP2 precludes binding to the IRE stem loop structure. A 32P-labeled Ft stem loop structure was used to assay IRP-binding from cell extracts of HFE expressing cell lines. Increases in the binding of IRPs to the IRE were detected in cells expressing fWTHFE and fW81AHFE (Dox-) over cells where the expression of HFE was suppressed (Dox+), indicating lower intracellular iron levels (Fig. 3B). Quantitation of the IRP/IRE bands indicated that the ratio of IRP/IRE complex in the cells expressing versus not expressing fWTHFE was 1.7. Similarly, the ratio was 1.5 in the cells expressing versus not expressing fW81AHFE. As expected, fC260YHFE cells showed no changes in intracellular iron whether or not the fC260YHFE was expressed (ratio = 0.95). Furthermore, decreases in intracellular Ft levels were detected by Western analysis when fWTHFE and fW81AHFE were induced by Dox withdrawal (Fig. 3C). In contrast, the induction of fC260YHFE had no effect on intracellular Ft levels consistent with its lack of function. These results are consistent with the low iron phenotype identified by gel-shift analysis and represent three independent tests for changes in the intracellular labile iron pool.
Fig. 3.
fW81AHFE/tTA HeLa cells have a low iron phenotype. (A) Tf-55Fe accumulation in cells. Tf-55Fe was added to the complete culture medium of cells expressing (Dox-) or not expressing (Dox+) HFE to the final concentrations of 25, 50, 100, 250, 500, 1,000, and 5,000 nM. Cells were incubated for 20 h at 37°C at 5% CO2. They were solubilized after acid wash at 4°C to strip off surface-bound Tf. The internal levels of 55Fe were expressed as pmol per 106 cells. Because of the similar uptake rates between fW81AfHFE/tTA and fWTHFE/tTA HeLa (Dox+), the results were averaged together and labeled Dox+. The experiment was repeated with similar results. (B) Gel-shift analysis of IRP binding to IREs by fWTHFE/tTA (WT), fW81AHFE/tTA (W81A), and fC260YHFE/tTA (C260Y) HeLa cell extracts. Extracts from cells expressing (Dox-) or not expressing (Dox+) HFE were incubated with 32P-labeled IRE as described in Experimental Methods and subjected to PAGE. Quantitative analysis of the IRE/IRP complexes in B was done by using a PhosphorImager. (C) Western blot of Ft levels using the same cell extracts as for IRP binding analysis in B. Thirty micrograms of lysate protein was loaded onto each lane. Blots were probed with an antibody to Ft as described in Experimental Methods. The experiments shown in B and C were repeated with similar results.
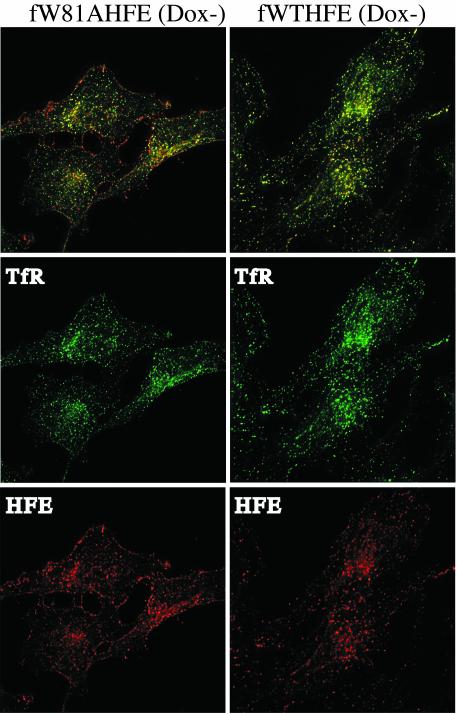
Subcellular Localization of fWTHFE and fW81AHFE. The fW81AHFE/tTA HeLa cells gave different results in Ft expression compared with HuTu80 cells expressing a similar construct (23), so we decided to analyze the localization of HFE in fW81AHFE/tTA HeLa cells under Dox- conditions by using immunofluorescence labeling. In the duodenal epithelial cell line, HuTu-80, fW81AHFE does not alter Ft levels and is exclusively found on the cell surface (23). In the fW81AHFE/tTA HeLa cell line, although fW81AHFE appeared extensively on the cell surface, it was also found in intracellular organelles positive for TfR (Fig. 4). In contrast, the fluorescent pattern in fWTHFE/tTA HeLa cells showed almost a complete overlap between HFE and TfR containing compartments and little fWTHFE unassociated with TfR on the cell surface. fW81AHFE could be entering the endocytic compartments via a nonconcentrative uptake system or by interaction with another protein possessing an endocytic signal. The differences in the distribution of HFE in the HeLa and HuTu-80 cells could explain the differences seen in the effect of the fW81A mutation of HFE on iron homeostasis in that internalization may be critical for the effect of HFE in lowering intracellular iron levels.
Fig. 4.
Localization of HFE and TfR in fW81AHFE and fWTHFE/tTA HeLa cells. Permeabilized cells were visualized with sheep anti-TfR, mouse anti-HFE, donkey anti-sheep (Alexa 488), and donkey anti-mouse (Alexa 594) antibodies as described in Experimental Methods.
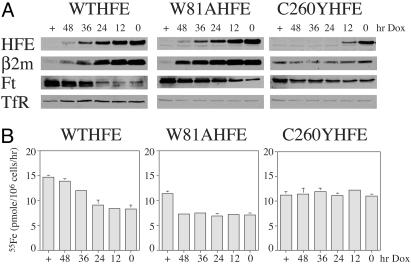
Levels of HFE Required to Reduce Intracellular Iron Levels. The amount of HFE required to inhibit Tf-mediated iron uptake was measured to determine the levels of HFE necessary to decrease intracellular iron levels and to determine whether HeLa cells could produce adequate amounts of β2m. A previous paper (24) presented evidence that lack of β2m expression resulted in decreased iron uptake into cells, whereas when β2m was expressed, more iron was taken up into cells. To alter the levels of HFE in cells, Dox was added to the culture medium of fW81AHFE, fC260YHFE, and fWTHFE/tTA HeLa cells to turn off their expression at different time points (Fig. 5A). The HFE protein levels are inversely correlated to the intervals of Dox inclusion in the medium with the highest at no Dox addition (0 h) and lowest with Dox present continuously (+). Visually, both fW81AHFE and fWTHFE show a similar half life. In contrast, fC260YHFE disappears quickly after the addition of Dox, indicating that this misfolded form of HFE is more rapidly degraded. In addition to HFE, the levels of β2m and Ft were also visualized to examine the iron status of the cells and determine whether β2m levels paralleled increases in HFE levels (Fig. 5A). Increases in β2m corresponded to increases in fW81AHFE or fWTHFE despite the fact that neither of these cell lines were transfected with β2m. This result could be due to the possibility that HFE expression itself could induce the expression of β2m. Alternatively, the expression of HFE and its association with β2m could result in more retention of β2m within the cells. Because β2m does not have a cytoplasmic domain, excess β2m would be secreted. In either case, HeLa cells appear to have sufficient amounts of β2m to complex with the transfected HFE. In contrast, cells that express the fC260YHFE show no measurable changes in β2m levels (Fig. 5A). At all levels of HFE expression, decreased Ft levels were observed in both fW81AHFE- and fWTHFE-expressing HeLa cells, but not in cells expressing the fC260YHFE.
Fig. 5.
The effect of the level of HFE expression on Tf-mediated iron uptake into cells. (A) Western blots of HFE, β2m, Ft, and TfR. Cell lines expressing HFE were treated for 0–48 h or maintained continuously with Dox (1 μg/ml) to turn off HFE expression. At each time point they were solubilized and subjected to Western analysis with the indicated antibodies. For all of the Western blots, 30 μg of protein was loaded into each lane. (B) Tf-55Fe uptake. Tf-55Fe uptake was measured after cells were incubated with Dox to turn off HFE expression for the same time points as in A. 55Fe-Tf (100 nM) was used. The results of the 1-h incubation at 37°C are presented for simplicity, although linear uptake was always observed for at least 3 h. The rates of uptake are expressed as pmol 55Fe per 106 cells per h. Nonspecific background was performed the same as above except that the incubation was performed on ice. Samples were always in triplicate and the experiment was repeated with similar results.
The amounts of Tf-mediated iron uptake at different levels of HFE were measured to quantitate the effect of HFE expression on the inhibition of iron uptake. Both fW81AHFE and fWTHFE expressing cell lines showed lowered amounts of Tf-mediated iron uptake (Fig. 5B). A striking difference between the decrease in Tf-mediated iron uptake is seen between the two cell lines. In fWTHFE/tTA HeLa cells, the extent of inhibition is proportional to the levels of HFE. In contrast, in fW81AHFE/tTA HeLa cells, Tf-mediated iron uptake remains depressed over a wide range of HFE levels. No change in Tf-mediated iron uptake was measured in fC260YHFE-transfected cells, consistent with the mutant HFE's lack of function. These results lead to the speculation that HFE interacts with another protein that is expressed at much lower concentrations in the cells than TfR. The binding of Tf to the TfR would release HFE from the TfR and allow it to interact with the hypothetical protein, perhaps the endosomal iron transporter.
Discussion
HFE down-regulates Tf-associated iron uptake and results in a low iron phenotype in a variety of cells (17, 23, 25–28). But the mechanism by which it down-regulates iron accumulation in cells is not well understood. Previous studies demonstrated that TfR has overlapping binding sites for both HFE and diferric Tf (15, 29) and HFE expression lowers the binding affinity of TfR to diferric Tf (13). We show that at low, subphysiological concentrations of circulating Tf (1–100 nM), Tf competed effectively with full-length HFE for binding to the TfR. However, at physiological levels of diferric Tf (10 μM), where no HFE would be expected to bind to TfR, we still detected an inhibition of Tf-mediated iron uptake into cells. Both the short-term and long-term Tf-associated Fe uptake studies showed that the inhibition by HFE is independent of Tf concentration. Essentially the same extent of inhibition was observed from a low concentration (100 nM) to its physiological concentration in the serum (≈10 μM). Coimmunoprecipitation studies also confirmed the previous finding that inhibition of Tf binding to TfR by HFE only occurs when the concentration of Tf is <100 nM. These data exclude the possibility that the inhibition of Tf-associated iron uptake is through the decreased binding of diferric Tf to TfR by HFE.
To further test the idea that HFE can act independently of TfR in decreasing iron uptake, new tTA HeLa cell lines with stably transfected fW81AHFE and fC260YHFE were generated by using the Tet-off regulatory system. The W81AHFE mutation was originally designed to interfere with the interaction of HFE and TfR but still associate with β2m and fold correctly. Plasmon resonance binding studies demonstrated that the binding affinity of W81AHFE to TfR is ≈5,000-fold lower than that of the WT HFE (21). In contrast, C260YHFE does not associate with either β2m or TfR. fW81AHFE exhibits essentially the same effects when expressed in cells as its WT counterpart, which includes a decrease in Tf-associated iron uptake and Ft levels, and increased IRP/IRE binding. When a correlation between the levels of HFE expression and the amount of Tf-mediated iron uptake were compared, we found a distinct and interesting difference between the fWTHFE- and fW81AHFE-transfected cells. The extent of Tf-mediated iron uptake was inversely proportional to the levels of HFE in fWTHFE transfected cells, whereas in fW81AHFE transfected cells, the same amount of low levels of Tf-mediated iron uptake was observed over a wide range of fW81AHFE expression. As a control, no effect of fC260YHFE on iron homeostasis in transfected cells was detected. These results, therefore, support the hypothesis that HFE down-regulation of iron homeostasis can be independent of its association with TfR.
Our present results are not completely consistent with a recent study in HuTu-80 cells (23). Like the HeLa cells, the expression of WT HFE in HuTu-80 cells decreased intracellular Ft levels. However, unlike our present results, no decrease in Ft levels was detected when W81A HFE was expressed in HuTu-80 cells. One possible cause for this discrepancy might be the difference between the nonpolarized cells (HeLa) and polarized duodenal epithelial cells (HuTu-80) in terms of differences in the targeting of HFE in different cell types. Alternatively, the cell lines may differ in the expression of key proteins that could interact with HFE. For example, divalent metal transporter 1 mRNA levels do not vary with iron loading in HeLa cells (A.-S.Z. and P.S.D., unpublished results) but do in other cell lines (30). HeLa cells show little iron efflux, whereas HuTu80 cells, being an intestinal epithelial cell line, would be expected to export iron from its basolateral surface.
Other cells and cell lines do not respond to HFE expression in the same way that HeLa cells do. In monocytes from HH patients, expression of HFE results in iron accumulation rather than depletion (31). Recent results of a monocytic cell line (THP1) transfected with HFE also indicates that expression of HFE results in iron accumulation (32). The latter study demonstrated that iron accumulation within these cells was caused by a decrease in the efflux of iron from the cells. We have demonstrated that HFE inhibits Tf-mediated iron uptake into HeLa cells. If a cell line like HeLa takes up iron and does not release it through an efflux mechanism, then expression of HFE would result in a low iron phenotype. On the other hand, in cell lines where there is a large flux of iron through the cell-like monocytes and differentiated enterocytes, the net effect of HFE would be to inhibit iron efflux. Alternatively, if the binding of Tf to the TfR releases HFE to bind to another partner, then HFE could have different binding partners in different cell types and thus affect either the influx or efflux of iron.
In conclusion, this study excludes the possibility that in HeLa cells HFE down-regulates Tf-associated iron uptake through competition of binding to TfR with diferric Tf. Instead, our results showed that HFE association with TfR is not required with respect to its function in the regulation of iron homeostasis. Therefore, we assume that HFE exerts its role through an uncharacterized partner(s). The challenge is to further identify the possible binding partners of HFE and to determine how HFE affects both Tf-mediated iron uptake and iron efflux.
Acknowledgments
We acknowledge the technical assistance of Emily Anderson and Sonya Votroubek, and we thank Martha Johnson for critical reading of the manuscript. This work was supported by National Institutes of Health Grant DK 54488. H.L.C. and P.S.D. were supported in part by National Institutes of Health Training Grants T32-HL00781 and 2T32-A107472, respectively.
Abbreviations: Tf, transferrin; TfR, Tf receptor; HH, hereditary hemochromatosis; Ft, ferritin; β2m, β2-microglobulin; IRP, iron regulatory protein; IRE, iron-responsive element; Dox, doxycyline.
References
- 1.Andrews, N. C. (1999) N. Engl. J. Med. 341, 1986-1995. [DOI] [PubMed] [Google Scholar]
- 2.Enns, C. A. (2001) Traffic 2, 167-174. [DOI] [PubMed] [Google Scholar]
- 3.Feder, J. N., Gnirke, A., Thomas, W., Tsuchihashi, Z., Ruddy, D. A., Basava, A., Dormishian, F., Domingo, R. J., Ellis, M. C., Fullan, A., et al. (1996) Nat. Genet. 13, 399-408. [DOI] [PubMed] [Google Scholar]
- 4.Feder, J. N., Tsuchihashi, Z., Irrinki, A., Lee, V. K., Mapa, F. A., Morikang, E., Prass, C. E., Starnes, S. M., Wolff, R. K., Parkkila, S., et al. (1997) J. Biol. Chem. 272, 14025-14028. [DOI] [PubMed] [Google Scholar]
- 5.Waheed, A., Parkkila, S., Zhou, X. Y., Tomatsu, S., Tsuchihashi, Z., Feder, J. N., Schatzman, R. C., Britton, R. S., Bacon, B. R. & Sly, W. S. (1997) Proc. Natl. Acad. Sci. USA 94, 12384-12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, X. Y., Tomatsu, S., Fleming, R. E., Parkkila, S., Waheed, A., Jiang, J., Fei, Y., Brunt, E. M., Ruddy, D. A., Prass, C. E., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 2492-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos, M., Schilham, M. W., Rademakers, L. H., Marx, J. J., de Sousa, M. & Clevers, H. (1996) J. Exp. Med. 184, 1975-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg, B. E. & Voland, J. R. (1996) Proc. Natl. Acad. Sci. USA 93, 1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy, J. E., Montross, L. K. & Andrews, N. C. (2000) J. Clin. Invest. 105, 1209-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oates, P. S., Trinder, D. & Morgan, E. H. (2000) Pflugers Arch. 440, 496-502. [DOI] [PubMed] [Google Scholar]
- 11.Trinder, D., Olynyk, J. K., Sly, W. S. & Morgan, E. H. (2002) Proc. Natl. Acad. Sci. USA. 99, 5622-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebron, J. A., Bennett, M. J., Vaughn, D. E., Chirino, A. J., Snow, P. M., Mintier, G. A., Feder, J. N. & Bjorkman, P. J. (1998) Cell 93, 111-123. [DOI] [PubMed] [Google Scholar]
- 13.Feder, J. N., Penny, D. M., Irrinki, A., Lee, V. K., Lebron, J. A., Watson, N., Tsuchihashi, Z., Sigal, E., Bjorkman, P. J. & Schatzman, R. C. (1998) Proc. Natl. Acad. Sci. USA 95, 1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waheed, A., Parkkila, S., Saarnio, J., Fleming, R. E., Zhou, X. Y., Tomatsu, S., Britton, R. S., Bacon, B. R. & Sly, W. S. (1999) Proc. Natl. Acad. Sci. USA 96, 1579-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West, A. P., Jr., Giannetti, A. M., Herr, A. B., Bennett, M. J., Nangiana, J. S., Pierce, J. R., Weiner, L. P., Snow, P. M. & Bjorkman, P. J. (2001) J. Mol. Biol. 313, 385-397. [DOI] [PubMed] [Google Scholar]
- 16.Gross, C. N., Irrinki, A., Feder, J. N. & Enns, C. A. (1998) J. Biol. Chem. 273, 22068-22074. [DOI] [PubMed] [Google Scholar]
- 17.Roy, C. N., Penny, D. M., Feder, J. N. & Enns, C. A. (1999) J. Biol. Chem. 274, 9022-9028. [DOI] [PubMed] [Google Scholar]
- 18.Warren, R. A., Green, F. A. & Enns, C. A. (1997) J. Biol. Chem. 272, 2116-2121. [DOI] [PubMed] [Google Scholar]
- 19.Warren, R. A., Green, F. A., Stenberg, P. E. & Enns, C. A. (1998) J. Biol. Chem. 273, 17056-17063. [DOI] [PubMed] [Google Scholar]
- 20.Eisenstein, R. S., Tuazon, P. T., Schalinske, K. L., Anderson, S. A. & Traugh, J. A. (1993) J. Biol. Chem. 268, 27363-37370. [PubMed] [Google Scholar]
- 21.Lebron, J. A. & Bjorkman, P. J. (1999) J. Mol. Biol. 289, 1109-1118. [DOI] [PubMed] [Google Scholar]
- 22.Parkkila, S., Waheed, A., Britton, R. S., Bacon, B. R., Zhou, X. Y., Tomatsu, S., Fleming, R. E. & Sly, W. S. (1997) Proc. Natl. Acad. Sci. USA 94, 13198-13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalingam, T. S., West, A. P., Jr., Lebron, J. A., Nangiana, J. S., Hogan, T. H., Enns, C. A. & Bjorkman, P. J. (2000) Nat. Cell Biol. 2, 953-957. [DOI] [PubMed] [Google Scholar]
- 24.Waheed, A., Grubb, J. H., Zhou, X. Y., Tomatsu, S., Fleming, R. E., Costaldi, M. E., Britton, R. S., Bacon, B. R. & Sly, W. S. (2002) Proc. Natl. Acad. Sci. USA 99, 3117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter-Cid, L., Brunmark, A., Li, Y., Leturcq, D., Peterson, P. A., Jackson, M. R. & Yang, Y. (1999) Proc. Natl. Acad. Sci. USA 96, 5434-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsi, B., Levi, S., Cozzi, A., Corti, A., Altimare, D., Albertini, A. & Arosio, P. (1999) FEBS Lett. 460, 149-152. [DOI] [PubMed] [Google Scholar]
- 27.Riedel, H. D., Muckenthaler, M. U., Gehrke, S. G., Mohr, I., Brennan, K., Herrmann, T., Fitscher, B. A., Hentze, M. W. & Stremmel, W. (1999) Blood 94, 3915-3921. [PubMed] [Google Scholar]
- 28.Feeney, G. P. & Worwood, M. (2001) Biochim. Biophys. Acta 1538, 242-251. [DOI] [PubMed] [Google Scholar]
- 29.Lebron, J. A., West, A. P., Jr., & Bjorkman, P. J. (1999) J. Mol. Biol. 294, 239-245. [DOI] [PubMed] [Google Scholar]
- 30.Gunshin, H., Allerson, C. R., Polycarpou-Schwarz, M., Rofts, A., Rogers, J. T., Kishi, F., Hentze, M. W., Rouault, T. A., Andrews, N. C. & Hediger, M. A. (2001) FEBS Lett. 509, 309-316. [DOI] [PubMed] [Google Scholar]
- 31.Montosi, G., Paglia, P., Garuti, C., Guzman, C. A., Bastin, J. M., Colombo, M. P. & Pietrangelo, A. (2000) Blood 96, 1125-1129. [PubMed] [Google Scholar]
- 32.Drakesmith, H., Sweetland, E., Schimanski, L., Edwards, J., Cowley, D., Ashraf, M., Bastin, J. & Townsend, A. R. (2002) Proc. Natl. Acad. Sci. USA 99, 15602-15607. [DOI] [PMC free article] [PubMed] [Google Scholar]