Abstract
Existing evidence indicates that, in addition to its neuroendocrine action, growth hormone-releasing hormone (GHRH) acts directly on several nonpituitary tissues, especially neoplasms, and stimulates cell proliferation. We have recently reported that a splice variant of the receptor (SV1) is expressed in various normal tissues and particularly in tumor tissues, producing mitogenic effects on GHRH binding. By using HEC-1A human endometrial carcinoma cells, which express endogenous SV1, we show that, in addition to its ability to mediate the mitogenic effects of GHRH, SV1 also possesses relatively high intrinsic, ligand-independent activity. By using an antisense RNA-based approach we found that SV1 ablation reduces the efficacy of colony formation and the rate of cell proliferation of HEC-1A cells in the absence of exogenous GHRH, and decreases their sensitivity to GHRH when the neurohormone is added to the culture media. This ligand-independent stimulation of cell proliferation appears to be a characteristic property of the truncated form of the receptor, because the expression of SV1 and not of the full-length GHRH receptor stimulated the proliferation of 3T3 fibroblasts in the absence of exogenous GHRH, whereas both forms mediated the proliferative effects of GHRH. Evaluation of 21 specimens of human primary endometrial carcinoma for expression of SV1 by immunohistochemistry indicated that in contrast to the GHRH receptor, which is absent, SV1 is expressed in ≈43% of the specimens. These findings indicate that SV1 can operate in a ligand-independent as well as a ligand-dependent manner. The overexpression of this form of GHRH receptor may be associated with carcinogenesis.
Growth hormone-releasing hormone (GHRH) is secreted by the hypothalamus and, after binding to specific GHRH receptors on the pituitary somatotrophs, it stimulates the release of growth hormone (GH) (1–3). GH in turn stimulates the production of insulin-like growth factor I, predominantly in the liver (2, 4). Insulin-like growth factor I is a mitogen for various cell types and has been linked with malignant transformation, tumor progression, and metastasis of various cancers (reviewed in ref. 5). In vitro and in vivo studies using specific antagonists of GHRH revealed that in addition to their action in suppressing cancer growth by interfering with the production of pituitary GH and hepatic insulin-like growth factor I, GHRH antagonists act directly on cancer cells and strongly inhibit their proliferation (4, 6–11). In some cells the proliferation is induced by the stimulatory action of local GHRH, which implies that GHRH may function as an autocrine/paracrine growth factor for various cancers such as small-cell lung carcinoma and ovarian cancer (5, 11).
Although the expression of the GHRH peptide has been detected in various normal and malignant human tissues, including cancer cell lines, the receptor for GHRH (GHRHR) was absent from virtually every human nonpituitary tissue tested with the exception of renal tissue (12–17). GHRHR was absent from tissues in which GHRH had been previously shown to produce autocrine stimulation of cell proliferation. GHRH, and presumably GHRH antagonists, could act through receptors homologous to the GHRHR such as those for vasoactive intestine peptide, pituitary adenylyl cyclase, and others (18, 19). Nevertheless recent evidence indicates that several splice variants (SVs) of GHRHR are expressed in nonpituitary tissues, including primary cancers and established cell lines, whereas the pituitary GHRHR is not present (20–27). One of these splice variants, named SV1, lacks only a portion of the extracellular part of the full-length receptor and therefore represents a form of the receptor that possibly has a functional significance (21). Consistently with previous findings attributing an essential role to the transmembrane part of the GHRHR in ligand binding (28), we have demonstrated that the ectopic expression of SV1 in 3T3 fibroblasts confers ligand binding and restores the responsiveness of the cells to GHRH stimulation, as reflected by the rate of cell proliferation (29).
Because certain seven-transmembrane domain G-protein-coupled receptors can possess ligand-independent intrinsic activity we were interested in whether this could also be the case for the SV1 isoform of GHRHR (30–31). By using HEC-1A human endometrial carcinoma cells, which express SV1, we found that by inhibiting endogenous SV1 expression by using an antisense RNA-based approach, the ablation of the SV1, besides inducing the resistance to GHRH stimulation, also inhibits cell proliferation in the absence of GHRH. We also tested a bank of surgical specimens of human endometrial carcinoma for SV1 expression by immunocytochemistry and found that a subset of these specimens expresses SV1.
Materials and Methods
RNA Extraction and RT-PCR. Total RNA was isolated from cells by using the TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed as described (21). PCR amplification for SV1 (GenBank accession no. AF282259) expression was performed as follows. One microliter of cDNA was added to a 20-μl solution containing 1× PCR buffer, 1.8 mM MgCl2, 200 μM each dNTP, 50 ng of each primer, and 1.5 units of Taq DNA polymerase. The primers used were 5′-CCTACTGCCCTTAGGATGCTGG-3′ and 5′-CCCTTGCTCCTCCAGAGCATGG-3′, which amplify 1,371 bp of the SV1 sequence. PCR consisted of 1 cycle at 95°C for 3 min, and 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. PCR products were electrophoresed on 1.2% agarose gels, stained with ethidium bromide, and visualized under UV light.
Plasmid Construction. The construction of pcDNA3-SV1 was reported (30). pcDNA3-SV1 antisense (as) was made by amplifying the full-length SV1 (1,371 bp) from HEC-1A cells by RT-PCR and cloning it into the pCR-2.1 vector (Invitrogen) by using the TOPO-TA cloning kit (Invitrogen), following the manufacturer's instructions. Subsequently, the SV1 fragment that was inserted in the pCR-2.1 vector in the antisense orientation was excised as a KpnI–NotI fragment and subcloned in the pcDNA3 vector (Invitrogen), between the promoter of cytomegalovirus and the polyadenylation site of simian virus 40, by using standard cloning procedures. pcDNA3-GHRHR was constructed by subcloning the full-length GHRHR cDNA from HPR3C plasmid (a kind gift of K. Mayo, Northwestern University, Evanston, IL) into the pcDNA3 vector, by using standard cloning procedures. The pcDNA3 vector also contains the G418-resistance gene.
Cell Culture. NIH 3T3 mouse fibroblasts and HEC-1A human endometrial carcinoma cells were obtained from the American Type Culture Collection. 3T3 cells were cultured in DMEM and HEC-1A cells in McCoy's medium, both supplemented with antibiotics/antimycotics, and 10% FBS unless otherwise described, at 37°C in a humidified 95% air/5% CO2 incubator. Transfections were performed by seeding 5 × 104 cells in six-well tissue culture plates with 1 μg of the appropriate plasmid by using the Metafectene reagent (Biontex, Munich, Germany) according to the manufacturer's instructions. Stable lines were generated by selection of the resistant colonies with 500 μg/ml G418 (Invitrogen) for 2 weeks. During selection, culture media were changed daily. The rate of cell proliferation was calculated by seeding 104 (unless otherwise stated) cells in six-well-plates and subsequently counting them under a light microscope by using the trypan blue exclusion assay, at time points indicated. hGHRH(1–29)NH2 was obtained from A. F. Parlow (National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda).
Radioligand-Binding Studies. Radioiodinated derivatives of GHRH antagonist JV-1–42 were prepared by the chloramine-T method as described (23). Preparation of the membrane fractions from ≈3 × 108 HEC-1A endometrial cancer cells was carried out as reported (20, 23). Receptor binding of GHRH was performed by using in vitro ligand competition assays based on the binding of 125I-JV-1–42 to membrane fractions of HEC-1A cells as described in detail (20, 23). The characteristics of the specific ligand binding were determined with the LIGAND-PC computerized curve-fitting program and by Scatchard analysis.
Colony Formation Assay. As described above, 5 × 104 cells were transfected in triplicates with pcDNA3 or pcDNA3-SV1(as), transferred after 2 days in 10-cm tissue culture dishes, and subjected to G418 selection for 2 weeks. Subsequently, colonies were fixed in 2% neutralized buffered formalin, stained with hematoxylin, and counted. Only colonies consisting of at least 10–15 cells were scored.
Immunocytochemistry. Formalin-fixed paraffin-embedded endometrial cancer tissues, from 21 patients who had been surgically treated, were collected from the Surgical Pathology archives of the Department of Pathology, Aretaieion Hospital, University of Athens, Athens. The study was approved by the Hospital Ethics and Scientific Research Committee. All patients were treated by total extrafascial hysterectomy, bilateral salpingo-oophorectomy, and pelvic and paraaortic lymph node sampling. Patients were staged according to the Fédération Internationale de Gynécologie et d'Obstetrique classification, and their tumors were graded as well (G1), moderately (G2), or poorly (G3) differentiated. For immunohistochemistry 4-mm-thick sections were used. Serial sections from representative paraffin-embedded blocks were collected onto poly-L-lysine-coated slides and stained for GHRH antigen. The immunohistochemical detection of SV1 was performed with the rabbit anti-SV1 polyclonal antibody 2317/5 diluted with 1× PBS at 1:104, by using the Kwik-DAB kit (ThermoShandon, Pittsburgh) according to the manufacturer's instructions. The 2317/5 anti-SV1 antibody was designed and produced by J. Horvath, J. Varga, M. Zarandi, and K. Groot in the laboratory of one of us (A.V.S.); its development will be reported elsewhere. Specimens were evaluated for positive staining and classified according to the percentage of positive cells, into the following categories: 1–10%, ±; 11–30%, +; 31–70%, ++; and 71–100%, +++.
Results
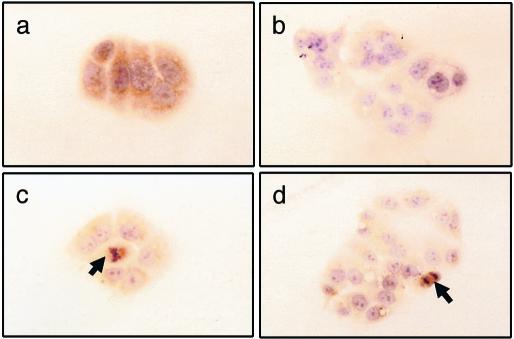
Development of HEC-1A Cells Expressing SV1(as). We performed our studies in HEC-1A endometrial carcinoma cells because they express SV1 as demonstrated by RT-PCR, immunoblotting, and immunocytological analysis (Fig. 1 and unpublished observations). HEC-1A cells were transfected with pcDNA3 (control) or pcDNA3-SV1(as). Stably transfected cells were pooled and harvested. As shown in Fig. 1b, immunocytological analysis using the 2317/5 rabbit polyclonal antibody raised against SV1 confirmed that the SV1(as) construct effectively inhibits endogenous SV1 expression; thus, this approach permits the study of the effects of SV1 ablation. We note, however, that, whereas the expression of SV1(as) inhibited the expression of SV1 in the vast majority of the cell population, some cells, especially those that undergo mitosis, were positive for anti-SV1 immunoreactivity (Fig. 1 c and d). Furthermore, long-term culture (>10 passages) of stably transfected HEC-1A cells resulted in the reappearance of SV1 staining. Whether the reappearance of SV1 staining is due to inadequate expression of SV1(as), loss of the plasmid, or specific overexpression of endogenous SV1 at levels above those that can be efficiently suppressed by the antisense construct requires investigation.
Fig. 1.
SV1 staining in HEC-1A endometrial carcinoma cells stably transfected with plasmids pcDNA3 (a) or pcDNA3-SV1(as) (b–d). Arrows in c and d indicate mitotic figures in Hec-1A cells, which show anti-SV1 immunoreactivity despite the presence of pcDNA3-SV1(as). Cells were counterstained with hematoxylin.
Radioligand Binding. By using complete-displacement analyses with 125I-labeled GHRH antagonist JV-1–42 as radioligand, we were able to detect specific, high-affinity (Kd = 1.77 ± 0.5 nM) binding sites for GHRH, with a maximal binding capacity of 169.3 ± 19.7 fmol/mg of membrane protein in membrane fraction of HEC-1A endometrial cancer cells.
Effect of SV1(as) on Cell Proliferation and Colony Formation. Initially we assessed the rate of proliferation of cells transfected with SV1(as). As shown in Fig. 2a, the cell number of pcDNA3-SV1(as)-transfected cells was ≈50% lower than that of the cells transfected with pcDNA3 alone (control), after 7 days of culture. The results of the colony-formation assay, which indicated that expression of pcDNA3-SV1(as) reduced the number of G418-resistant colonies by ≈55% compared with controls (P < 0.05) (Fig. 3), were consistent with the findings on inhibition of cell proliferation. However, no difference in the morphology of the colonies was found.
Fig. 2.
SV1 ablation by expression of pcDNA3-SV1(as) suppresses the rate of cell proliferation of HEC-1A endometrial carcinoma cells in both the absence and the presence of GHRH. Growth rate of Hec-1A cells transfected with plasmids pcDNA3 or pcDNA3-SV1(as), cultured in the absence of exogenously added GHRH in media containing 10% FBS (a), or in media containing 1% FBS with the addition of GHRH(1–29)NH2 at the concentrations indicated (b). Average values of experiments performed in triplicate are shown. *, P < 0.05 vs. cells transfected with the vector pcDNA3-SV1(as).
Fig. 3.
Suppression of colony formation in HEC-1A endometrial carcinoma cells by pcDNA3-SV1(as). (a) Representative Petri dishes of cells transfected with pcDNA3 or pcDNA3-SV1(as). The experiment was repeated in triplicate and average values are shown in b. *, P < 0.05 vs. controls.
Effect of hGHRH on the Proliferation of SV1(as)-Expressing Cells. Subsequently we evaluated how the inhibition of SV1 expression by SV1(as) affects the response of HEC-1A cells to stimulation with GHRH in terms of cell proliferation. Thus, 3 × 104 cells were cultured in media supplemented with 1% FBS in the presence of 0.01, 0.1, and 1 μM hGHRH(1–29)NH2 or in its absence, and the cell number was assessed after 6 days. As shown in Fig. 2b, the exposure to GHRH at 0.1 and 1 μM significantly (P < 0.05) stimulated, by ≈30% and 40%, respectively, the rate of cell proliferation of the control HEC-1A cells but had no effect in the cells expressing SV1(as). Some stimulation of ≈20% was noted in the pcDNA3-SV1(as)-expressing cells at 1 μM GHRH, but it was not significant.
Assessment of GHRH-Dependent and -Independent Effects of SV1 and GHRHR in 3T3 Fibroblasts. We then attempted to compare the GHRH-dependent and -independent effects of SV1 and full-length GHRHR. Thus we introduced these receptors into 3T3 fibroblasts, which express neither of them endogenously, and evaluated the rate of cell proliferation in the presence and absence of GHRH. When cells were cultured for 5 days in media containing 1% FBS, but without exogenously added GHRH, cells transfected with SV1 proliferated ≈50% faster than controls (P < 0.05), whereas cells transfected with GHRHR showed no significant difference in their proliferation rate (Fig. 4). However, when cells were cultured in the presence of GHRH, both SV1- and GHRHR-transfected cells were sensitive to the hormone, with the latter exhibiting a greater response. GHRHR-transfected cells showed a stimulation of cell proliferation by ≈60% (P < 0.05) in response to 0.1 μM GHRH; this stimulation was increased further to ≈80% (P < 0.05) with 1 μM GHRH. SV1-transfected cells exhibited significant stimulation of cell proliferation by ≈30% (P < 0.05), only with 1 μM GHRH, whereas pcDNA3-transfected cells showed a minimal but insignificant stimulation of cell proliferation at the concentrations of GHRH used (Fig. 4). Fig. 4b shows the percentage changes in the cell number vs. corresponding controls cultured in the absence of GHRH.
Fig. 4.
Effect of SV1 and GHRHR on the proliferation of 3T3 fibroblasts cultured in the absence or the presence of 0.1 and 1.0 μM hGHRH(1–29)NH2. Graphs show absolute cell number (a) or percent cell number vs. corresponding controls (cultured in the absence of GHRH) (b). Both SV1 and GHRHR mediate the mitogenic effects of GHRH, but only SV1 stimulates cell proliferation in the absence of exogenously added ligand. Average values of experiments performed in triplicate are shown. *, P < 0.05 vs. controls (cells transfected with the same plasmid but cultured in the absence of GHRH); **, P < 0.05 vs. cells transfected with pcDNA3.
Expression of SV1 in Primary Endometrial Carcinoma. Finally, we investigated in the cytopathology laboratory in Athens whether SV1 is expressed in primary endometrial tumors. A bank of 21 endometrial adenocarcinomas, consisting of 16 endometrioid tumors, three serous-papillary tumors, one mixed type endometrioid/serous-papillary tumor, and one malignant mixed mullerian tumor, were assessed for anti-SV1 immunoreactivity by using antibody 2317/5. As shown in Table 1, 9 (43%) specimens exhibited moderate (+), high (++), or intense (+++) immunoreactivity, which was localized in the cytoplasm of the epithelial cells of the glands of the endometrial adenocarcinomas (Fig. 5). No association between anti-SV1 immunoreactivity, the age of the patients, the grade and the extent of the myometrial invasion was found (Table 1). Some samples positive for anti-SV1 staining were assessed by using an antibody raised specifically for GHRHR, but no immunoreactivity was detected (data not shown).
Table 1. Clinicopathological data and expression of GHRHR splice variant SV1 in human endometrial adenocarcinoma specimens.
| Case no. | Age, yr | Histology* | Grade (FIGO)† | Myometrial invasion, % | SV1 expression |
|---|---|---|---|---|---|
| 1 | 50 | E | G2 | 100 | + |
| 2 | 53 | E | G2 | 80 | - |
| 3 | 70 | E | G1 | 100 | - |
| 4 | NA | E | G1-G2 | 50 | - |
| 5 | 60 | E | G1 | 33 | + |
| 6 | 64 | E | G1 | 67 | ++ |
| 7 | 72 | E | G1 | 25 | ++ |
| 8 | 54 | E+SP | G1 | 33 | - |
| 9 | 64 | E | G1 | 33-50 | - |
| 10 | 43 | E | G1 | 0 | +++ |
| 11 | 67 | E | G2 | 50 | ++ |
| 12 | 87 | E | G2 | 25-33 | - |
| 13 | 90 | MMMT | NA | 100 | +++ |
| 14 | 68 | SP | NA | 67-75 | ++ |
| 15 | 49 | E | G2 | 50 | - |
| 16 | 69 | SP | G2 | 33 | ± |
| 17 | 67 | E | G2 | 83 | + |
| 18 | 66 | SP | G3 | NA | - |
| 19 | 73 | E | G2 | 50 | - |
| 20 | 59 | E | G1 | 50 | ± |
| 21 | 53 | E | G2 | 0 | ± |
NA, not available
E, endometrioid; SP, serous papillary; MMMT, mixed mullerian tumor
FIGO, Fédération Internationale de Gynécologie et d'Obstetrique
Fig. 5.
Anti-SV1 immunoreactivity in primary endometrial carcinoma of the endometrioid (a–c) or serous-papillary (d) type. Cytoplasmic staining is apparent in the epithelial cells of the glands, whereas stromal cells are negative. Arrows indicate areas of immunopositivity. Specimens were counterstained with hematoxylin. (×40.) This study was performed in the cytopathology laboratory of Aretaieion University Hospital in Athens.
Discussion
Hypothalamic GHRH regulates the production and release of GH from the pituitary gland. The initial step in the action of GHRH is the binding to its receptors. The receptors for GHRH are expressed predominantly in the pituitary. Despite the tissue-specific pattern of expression of GHRHR, recent evidence demonstrates that splice variants of this receptor are expressed in several nonpituitary tissues, both normal and tumoral, as well as in cancer cell lines (2, 6, 8, 10, 20–25). Among these SVs of GHRHR is SV1, which lacks a portion of the extracellular part of the receptor but retains the ability to bind GHRH and to stimulate cell proliferation as well as cAMP production and release on ligand binding (20, 21, 29). If the only mode of action of SV1 was the mediation of GHRH binding, we expect that antagonistic analogs of GHRH would be inactive in the absence of GHRH. However, some evidence suggests that various antagonists of GHRH with weak inhibitory activity on the pituitary strongly inhibit the in vitro proliferation of cancer cells expressing SV1 (4, 6–10). Thus we hypothesized that besides the ligand-dependent activity, SV1 could also possess ligand-independent activity. We have previously shown that ligand-dependent activity can be nullified or reduced in the presence of GHRH antagonists. Now we wished to extend this hypothesis to ligand-independent activity. To test it, instead of evaluating the effects of SV1 blockade using specific antagonists, we decided to test the consequences of the inhibition of the endogenous SV1 production.
Consequently, after establishing that HEC-1A endometrial carcinoma cells express SV1 and display highly specific binding to GHRH, we constructed a plasmid that expresses the cDNA of SV1 in the antisense orientation. Previous studies have demonstrated that the expression of antisense RNA is an effective method for suppressing the expression of specific transcripts (32). The introduction of pcDNA3-SV1(as) into HEC-1A cells resulted in a considerable suppression of SV1 expression. We noted that in mitotic cells, in which the spindle formation is apparent, some anti-SV1 immunoreactivity was detectable. A possible explanation for this finding is that the levels of SV1 expression vary during cell cycle with cells during mitosis expressing higher levels of SV1, most likely above the threshold, that can be efficiently suppressed by the SV1(as) expression. This hypothesis has to be validated by further studies.
Subsequently, we evaluated the growth properties of the HEC-1A cells in the absence of SV1 expression. Colony formation assay indicated that the expression of pcDNA3-SV1(as) reduced the incidence of colonies that were resistant to antibiotic selection, as compared with cells transfected with empty vector. In accord with this proliferative role of SV1 in HEC-1A endometrial carcinoma, stably expressing SV1(as) cells exhibited a reduced rate of cell proliferation in the absence of exogenously added GHRH. Although these findings show that the inhibition of SV1 expression suppresses cell proliferation in the absence of exogenously added GHRH, the exposure of SV1(as)-transfected and control cells to GHRH and the subsequent evaluation of the cell proliferation rate indicated that the latter cells are significantly more sensitive to GHRH stimulation. HEC-1A cells expressing SV1(as) responded only to high doses of GHRH, probably because of nonspecific binding of this neuropeptide to other homologous receptors.
In the light of these findings, which confirm the ability of SV1 to mediate the proliferative effects of GHRH, it could be argued that the suppression of cell proliferation produced by SV1(as) is still ligand-dependent, by a mechanism involving the disruption of autocrine loop(s) operating between SV1 and GHRH or other similar peptides produced by the HEC-1A cells and released into the culture medium. In view of our findings (33) that HEC-1A express GHRH, this possibility cannot be fully excluded by the results of the experiments described herein. It seems unlikely, however, because during the colony-formation assay the culture medium was changed daily, thus preventing the accumulation of peptides secreted by the cells. We also questioned whether this property of SV1 to induce cell proliferation in the absence of GHRH is limited to the splice variant of the receptor or whether it is also a feature of the full-length GHRHR. Therefore we introduced SV1 and GHRHR into 3T3 fibroblasts and compared the rate of cell proliferation. We found that whereas both forms of the receptor conferred the responsiveness to GHRH, with GHRHR being more potent than SV1, the cells transfected with the latter proliferated faster than those expressing the full-length receptor. Thus we concluded that the high intrinsic proliferative activity is a feature of only the truncated receptor. It appears that in SV1 the deletion of a portion of the extracellular part of the protein does not greatly affect the ligand-binding properties of the receptor and its ability to elicit mitogenic signals in the presence of GHRH, but introduces conformational changes that result in a form of the receptor that is partially activated even in the absence of the bound ligand. Therefore in the absence of GHRH, SV1 has some activity, which, however, can increase further after ligand binding.
Finally, we investigated whether SV1 is expressed in primary human tumors, such as endometrial tumors. Using a polyclonal antibody raised specifically for SV1, we found that 43% of primary endometrial carcinoma specimens exhibited a moderate to intense anti-SV1 immunoreactivity, localized in the cytoplasm. Recently we reported that endometrial carcinoma specimens also express GHRH, and suggested the operation of an autocrine stimulatory loop between GHRH and SV1 (34). A similar stimulatory autocrine interaction between SV1 and GHRH has also been reported in prostatic carcinoma (22–24).
The generation of activated forms of hormone and other receptors by alternative splicing has been described before, for example, a splice variant of the mineralocorticoid receptor that abolishes the ligand-binding domain and acquires ligand-independent transactivation activity (35, 36). Estrogen receptor α has also been reported to undergo alternative splicing resulting in a form of the receptor, which synergizes with the full-length form of the receptor to mediate estrogen-dependent transcription (37). The receptor for pituitary adenylyl cyclase, which is highly homologous to the GHRHR, can be also subjected to alternative splicing encoding isoforms with differential properties of ligand binding and specificities (38).
Altogether, our work shows that SV1 can mediate mitogenic effects by both ligand-dependent and ligand-independent mechanism(s). In the absence of GHRH binding and stimulation, SV1 may elicit mitogenic signals, which can reach maximal potency only on ligand binding. The finding that SV1 is overexpressed in a subset of human endometrial cancers and probably in other tumors implies a role for this SV1 receptor in the development of malignancies. However, the precise role of SV1 in carcinogenesis as well as in physiological conditions still remains to be elucidated.
Acknowledgments
We are grateful to Dr. S. Artavanis-Tsakonas for help at the initial stages of the study. We also thank Dr. A. F. Parlow and the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, for hGHRH and Dr. K. Mayo for the HPR3C plasmid. The work of A.V.S. was supported by the Medical Research Service of the Department of Veterans Affairs and by a grant from Zentaris (Frankfurt, Germany) through Tulane University (to A.V.S.).
Abbreviations: GH, growth hormone; GHRH, GH-releasing hormone; hGHRH, human GHRH; GHRHR, GHRH receptor; SV, splice variant; SV1(as), SV1 antisense.
References
- 1.Schally, A. V. & Comaru-Schally A. M. (1998) in Growth Hormone Secretagogues in Clinical Practice, eds. Bercu, B. B. & Walker, R. F. (Dekker, New York), pp. 14-31.
- 2.Schally, A. V., Comaru-Schally, A. M., Nagy, A., Kovacs, M., Szepeshazi, K., Plonowski, A., Varga, J. L. & Halmos, J. (2001) Front. Neuroendocrinol, 22, 228-291. [DOI] [PubMed] [Google Scholar]
- 3.Frohman, L. A. & Kineman, R. D. (2002) Trends Endocrinol. Metab. 13, 299-303. [DOI] [PubMed] [Google Scholar]
- 4.Schally, A. V. & Varga, J. L. (1999) Trends Endocrinol. Metab. 10, 383-391. [DOI] [PubMed] [Google Scholar]
- 5.Furstenberger, G. & Senn, H. J. (2002) Lancet Oncol. 3, 298-302. [DOI] [PubMed] [Google Scholar]
- 6.Busto, R., Schally, A. V., Varga, J. L., Garcia-Fernandez, M. O., Groot, K., Armatis, P. & Szepeshazi, K. (2002) Proc. Natl. Acad. Sci. USA 99, 11866-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiaris, H., Schally, A. V., Varga, J. L, Groot, K. & Armatis, P. (1999) Proc. Natl. Acad. Sci. USA 96, 3098-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Busto, R., Armatis, P. & Halmos G. (2001) Anticancer Drugs 12, 761-768. [DOI] [PubMed] [Google Scholar]
- 9.Braczkowski, R., Schally, A. V., Plonowski, A., Varga, J. L., Groot, K., Krupa, M. & Armatis, P. (2002) Cancer 95, 1735-1745. [DOI] [PubMed] [Google Scholar]
- 10.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Armatis, P., Busto, R. & Halmos, G. (2001) J. Clin. Endocrinol. Metab. 86, 2144-2152. [DOI] [PubMed] [Google Scholar]
- 11.Zeitler, P. & Siriwardana, G. (2002) Endocrine 18, 85-90. [DOI] [PubMed] [Google Scholar]
- 12.Mayo, K. E. (1992) Mol. Endocrinol. 6, 1734-1744. [DOI] [PubMed] [Google Scholar]
- 13.Gaylinn, B. D., Harrison, J. K., Zysk, J. R., Lyons, C. E., Lynch, K. R. & Thorner, M. O. (1993) Mol. Endocrinol. 7, 77-84. [DOI] [PubMed] [Google Scholar]
- 14.Mayo, K. E., Miller, T. L., De Almeida, V., Zheng, J. & Godfrey, P. A. (1996) Ann. N. Y. Acad. Sci. 805, 184-203. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara, S., Makoto, S., Mizobuchi, M., Niimi, M. & Takahara, J. (1995) Endocrinology 136, 4147-4150. [DOI] [PubMed] [Google Scholar]
- 16.Boulanger, L., Girard, N., Strecko, J. & Gaudreau, P. (2002) Peptides (Tarrytown, NY) 23, 1187-1194. [DOI] [PubMed] [Google Scholar]
- 17.Boisvert, P., Pare, C., Veyrat-Durebex, C., Robert, A., Dubuisson, S., Morel, G. & Gaudreau, P. (2002) Endocrinology 143, 1475-1484. [DOI] [PubMed] [Google Scholar]
- 18.Sherwood, N. M., Krueckl, S. L. & McRory, J. E. (2000) Endocr. Rev. 21, 619-670. [DOI] [PubMed] [Google Scholar]
- 19.Maruno, K., Absood, A. & Said, S. I. (1998) Proc. Natl. Acad. Sci. USA 95, 14373-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halmos, G., Schally, A. V., Varga, J. L., Plonowski, A., Rekasi, Z. & Czompoly, T. (2000) Proc. Natl. Acad. Sci. USA 97, 10555-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rekasi, Z., Czompoly, T., Schally, A. V. & Halmos, G. (2000) Proc. Natl. Acad. Sci. USA 97, 10561-10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopin, L. K. & Herington, A. C. (2001) Prostate 49, 116-121. [DOI] [PubMed] [Google Scholar]
- 23.Halmos, G., Schally, A. V., Czompoly, T., Krupa, M., Varga, J. L. & Rekasi, Z. (2002) J. Clin. Endocrinol. Metab. 87, 4707-4714. [DOI] [PubMed] [Google Scholar]
- 24.Plonowski, A., Schally, A. V., Busto, R., Krupa, M., Varga, J. L. & Halmos, G. (2002) Peptides (Tarrytown, NY) 23, 1127-1133. [DOI] [PubMed] [Google Scholar]
- 25.Busto, R., Schally, A., Braczkowski, R., Plonowski, A., Krupa, M., Groot, K., Armatis, P. & Varga, J. (2002) Regul. Pept. 108, 47-53. [DOI] [PubMed] [Google Scholar]
- 26.Plonowski, A., Schally, A. V., Letsch, M., Krupa, M., Hebert, F., Busto, R., Groot, K. & Varga, J. L. (2002) Prostate 52, 173-182. [DOI] [PubMed] [Google Scholar]
- 27.Kahan, Z., Varga, J. L., Schally, A. V., Rekasi, Z., Armatis, P., Chatzistamou, I., Czompoly, T. & Halmos, G. (2000) Breast Cancer Res. Treat. 60, 71-79. [DOI] [PubMed] [Google Scholar]
- 28.DeAlmeida, V. I. & Mayo, K. E. (1998) Mol. Endocrinol. 12, 750-765. [DOI] [PubMed] [Google Scholar]
- 29.Kiaris, H., Schally, A. V., Busto, R., Halmos, G., Artavanis-Tsakonas, S. & Varga, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopin, A. S., McBride, E. W., Schaffer, K. & Beinborn, M. (2000) Trends Pharmacol. Sci. 21, 346-353. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick, G. J. Dautzenberg, F. M., Martin, G. R. & Eglen, R. M. (1999) Trends Pharmacol. Sci. 20, 294-301. [DOI] [PubMed] [Google Scholar]
- 32.Sazani, P., Vacek, M. M. & Kole, R. (2002) Curr. Opin. Biotechnol. 13, 468-472. [DOI] [PubMed] [Google Scholar]
- 33.Kahan, Z., Arencibia, J. M., Csernus, V. J., Groot, K., Kineman, R. D., Robinson, W. R. & Schally, A. V. (1999) J. Clin. Endocrinol. Metab. 84, 582-589. [DOI] [PubMed] [Google Scholar]
- 34.Chatzistamou, I., Schally, A. V., Pafiti, A., Kiaris, H. & Koutselini, H. (2002) Eur. J. Endocrinol. 147, 381-386. [DOI] [PubMed] [Google Scholar]
- 35.Zennaro, M. C., Souque, A., Viengchareun, S., Poisson, E. & Lombes, M. (2001) Mol. Endocrinol. 15, 1586-1598. [DOI] [PubMed] [Google Scholar]
- 36.Wickert, L., Watzka, M., Bolkenius, U., Bidlingmaier, F. & Ludwig, M. (1998)Eur. J. Endocrinol. 138, 702-704. [DOI] [PubMed] [Google Scholar]
- 37.Chaidarun, S. S. & Alexander, J. M. (1998) Mol. Endocrinol. 12, 1355-1366. [DOI] [PubMed] [Google Scholar]
- 38.Dautzenberg, F. M., Mevenkamp, G., Wille, S. & Hauger, R. L. (1999) J. Neuroendocrinol. 11, 941-949. [DOI] [PubMed] [Google Scholar]