Abstract
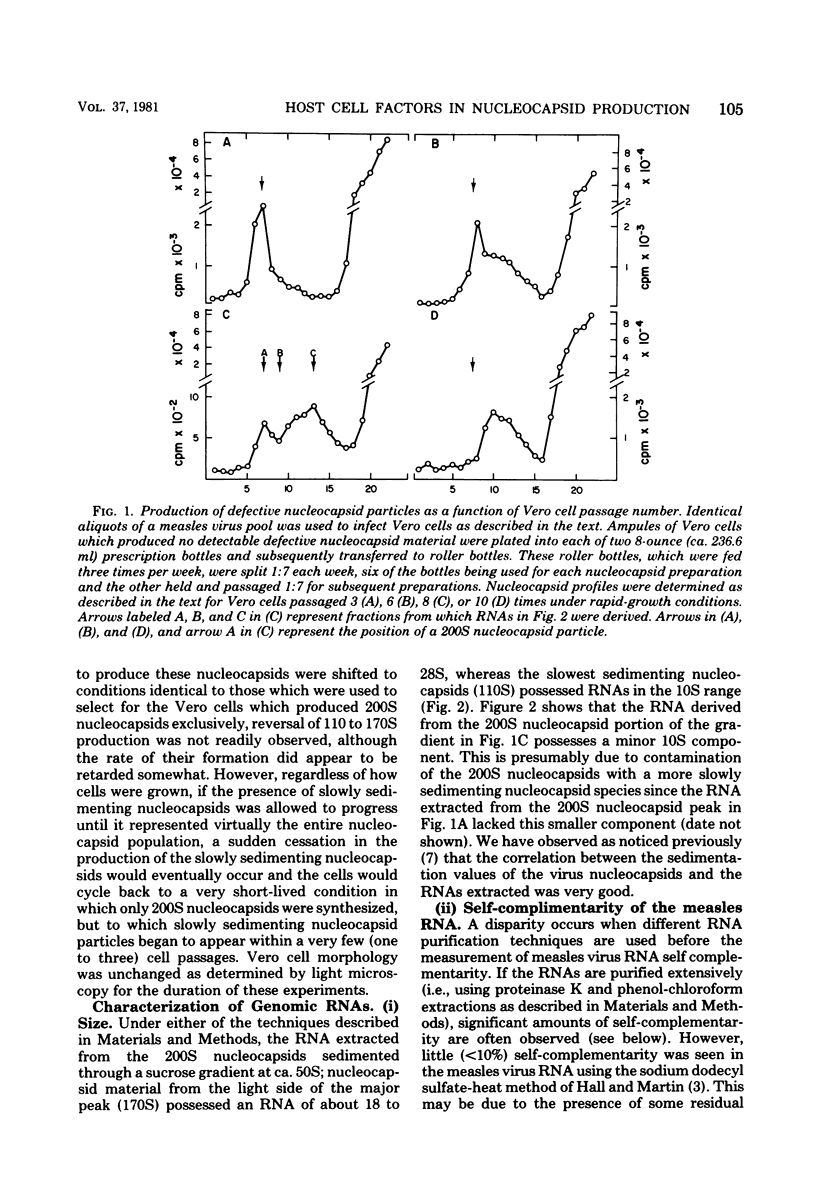
A decrease in the sedimentation rates of the measles virus nucleocapsid, and the RNA contained within, were observed during acute measles virus infection when the growth conditions of Vero cells were altered. The change in sedimentation rates of virus nucleocapsids in these experiments was apparently due to the physiological state of the cell and was independent of the history of the measles virus used for infection since: (i) the same virus stock was used to infect cells from which nucleocapsids were prepared, (ii) nucleocapsid sedimentation rates were rapid when Vero cells freshly revived from liquid nitrogen were infected, but nucleocapsid profiles showed no decrease in the amount of slowly sedimenting material using the same cells and changing the virus preparation used for infection. Frequent cell splittings and numerous medium changes were among the growth factors which appeared to correlate to slowly sedimenting particle production. Changes in the amount of self-complementarity of the measles virus RNA were also observed under these conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody J. A., Sever J. L., Edgar A., McNew J. Measles antibody titers of multiple sclerosis patients and their siblings. Neurology. 1972 May;22(5):492–499. doi: 10.1212/wnl.22.5.492. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Pons M. W. The RNAs of infective and incomplete influenza virions grown in MDBK and HeLa cells. Virology. 1970 Nov;42(3):603–610. doi: 10.1016/0042-6822(70)90306-5. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Jackson S. H. The metabolic effects of halothane on mammalian hepatoma cells in vitro. II. Inhibition of DNA synthesis. Anesthesiology. 1973 Oct;39(4):405–409. doi: 10.1097/00000542-197310000-00013. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. P., Gray R. H., Payne F. E. Replication of measles virus: distinct species of short nucleocapsids in cytoplasmic extracts of infected cells. J Virol. 1974 Mar;13(3):721–728. doi: 10.1128/jvi.13.3.721-728.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A. On the genesis of incomplete Sendai virions. Virology. 1970 Dec;42(4):872–879. doi: 10.1016/0042-6822(70)90336-3. [DOI] [PubMed] [Google Scholar]
- Knight P. R., Nahrwold M. L., Bedows E. Anesthetic action and virus replication: inhibition of measles virus replication in cells exposed to halothane. Antimicrob Agents Chemother. 1980 May;17(5):890–896. doi: 10.1128/aac.17.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Holland J. J., Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980 Jan 30;100(2):408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrwold M. L., Cohen P. J. Anesthetics and mitochondrial respiration. Clin Anesth. 1975;11(1):25–44. [PubMed] [Google Scholar]
- Perrault J. Cross-linked double-stranded RNA from a defective vesicular stomatitis virus particle. Virology. 1976 Apr;70(2):360–371. doi: 10.1016/0042-6822(76)90278-6. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Davidson W. B., Martin S. J. The role of defective interfering particles in persistent infection of Vero cells by measles virus. J Gen Virol. 1977 Apr;35(1):89–97. doi: 10.1099/0022-1317-35-1-89. [DOI] [PubMed] [Google Scholar]
- Rima R. K., Martin S. J. Persistent infection of tissue culture cells by RNA viruses. Med Microbiol Immunol. 1976 Jun 1;162(2):89–119. doi: 10.1007/BF02121320. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Bennett P. B., Shrivastav B. B. Interaction of halothane with lipid bilayers. Mol Pharmacol. 1979 Jul;16(1):163–170. [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]


