Abstract
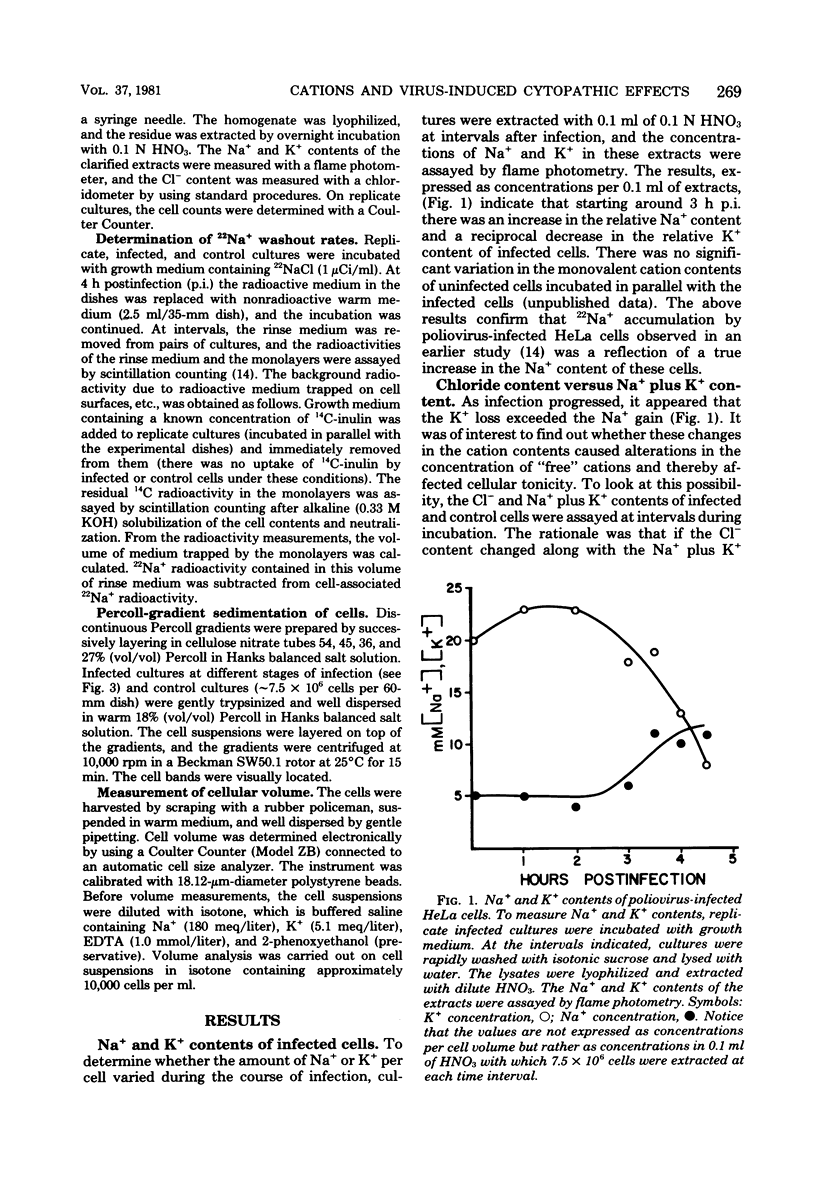
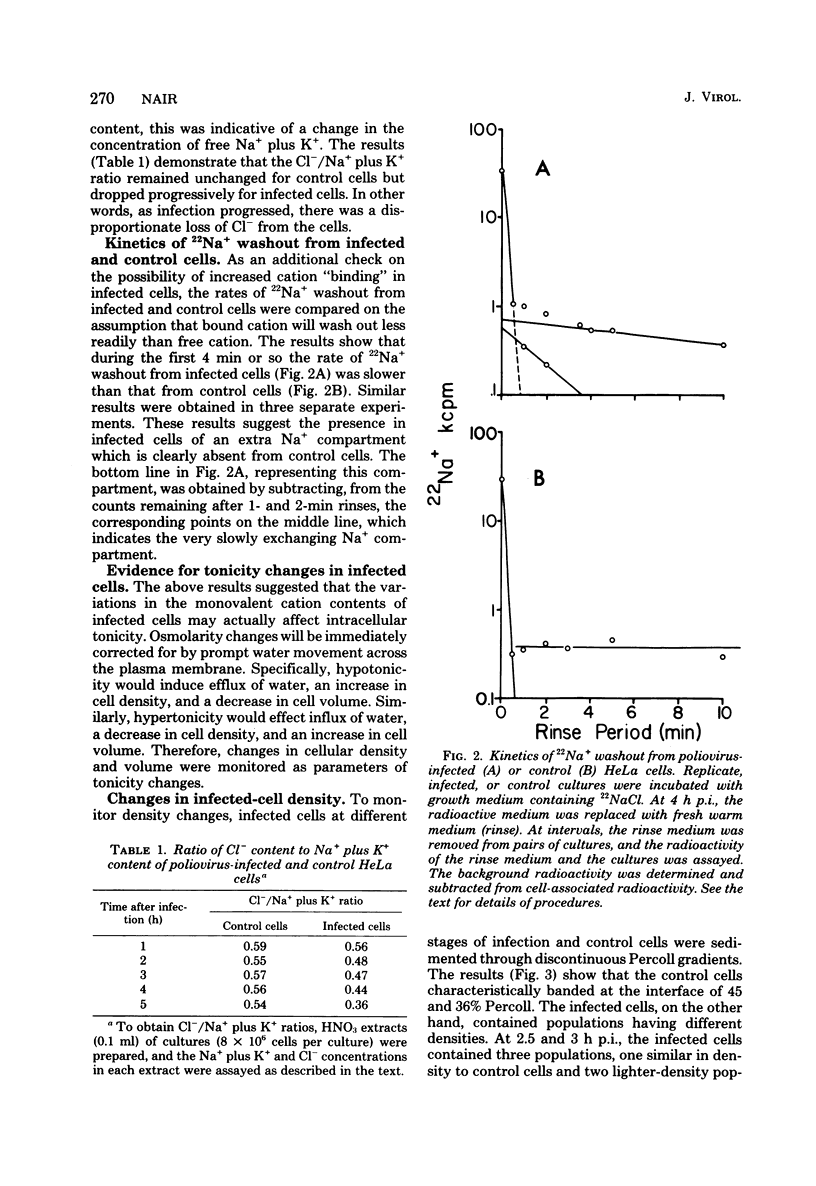
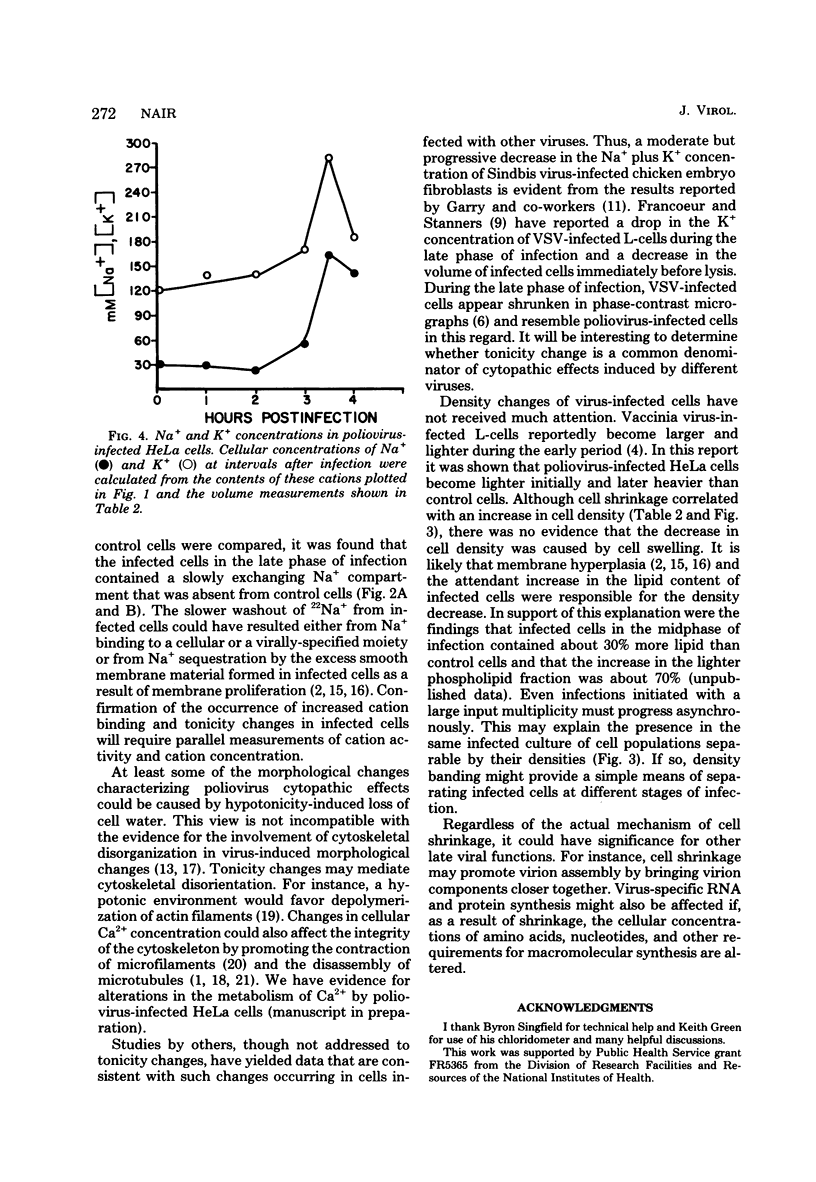
To better understand the significance of 22Na+ accumulation by poliovirus-infected HeLa cells (C. N. Nair, J. W. Stowers, and B. Singfield, J. Virol. 31:184, 1979), measurements of cellular Na+, K+, and Cl- contents, volume, and density were carried out at intervals after infection. In addition, the rates of 22Na+ washout from infected and control cells were determined. Starting at around 3 h postinfection, the Na+ content of infected cells increased, whereas the K+ content decreased progressively, resulting in a net loss in the monovalent cation content decreased progressively, resulting in a net loss in the monovalent cation content per cell. The loss in cellular chloride content exceeded that in monovalent cation content. The kinetics of 22Na+ washout from infected and control cells revealed the presence of an extra Na+ compartment in infected cells. A net loss in the monovalent cation activity of infected cells was indicated by the loss of cell water as reflected in a decrease in cell volume and an increase in cell density. In spite of a net loss in monovalent cation content per cell, Na+ accumulation coupled with cell shrinkage resulted in substantial increases in the concentrations of not only Na+ but also K+. The results suggested a possible role for tonicity change in the morphological lesions of poliovirus cytotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. The role of microfilaments and microtubules in cell movement, endocytosis and exocytosis. Ciba Found Symp. 1973;14:109–148. doi: 10.1002/9780470719978.ch6. [DOI] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Balanian R. Structural and functional alterations in cultured cells infected with cytocidal viruses. Prog Med Virol. 1975;19:40–83. [PubMed] [Google Scholar]
- Ball F. R., Medzon E. L. Sedimentation changes of L cells in a density gradient early after infection with vaccinia virus. J Virol. 1973 Sep;12(3):588–593. doi: 10.1128/jvi.12.3.588-593.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Sodium ions and the shut-off of host cell protein synthesis by picornaviruses. Nature. 1976 Dec 23;264(5588):807–809. doi: 10.1038/264807a0. [DOI] [PubMed] [Google Scholar]
- David-West T. S., Osunkoya B. O. Cytopathology of vesicular stomatitis virus with phase microscopy. Arch Gesamte Virusforsch. 1971;35(1):126–132. doi: 10.1007/BF01249759. [DOI] [PubMed] [Google Scholar]
- Egberts E., Hackett P. B., Traub P. Alteration of the intracellular energetic and ionic conditions by mengovirus infection of Ehrlich ascites tumor cells and its influence on protein synthesis in the midphase of infection. J Virol. 1977 Jun;22(3):591–597. doi: 10.1128/jvi.22.3.591-597.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARNHAM A. E., EPSTEIN W. THE INFLUENCE OF ENCEPHALOMYOCARDITIS (EMC) VIRUS INFECTION ON POTASSIUM TRANSPORT IN L CELLS. Virology. 1963 Nov;21:436–447. doi: 10.1016/0042-6822(63)90205-8. [DOI] [PubMed] [Google Scholar]
- Francouer A. M., Stanners C. P. Evidence against the role of K+ in the shut-off of protein synthesis by vesicular stomatitis virus. J Gen Virol. 1978 Jun;39(3):551–553. doi: 10.1099/0022-1317-39-3-551. [DOI] [PubMed] [Google Scholar]
- Fuchs P., Giberman E. Enhancement of potassium influx, in baby hamster kidney cells and chicken erythrocytes, during adsorption of parainfluenza 1 (Sendai) virus. FEBS Lett. 1973 Apr 1;31(1):127–130. doi: 10.1016/0014-5793(73)80089-4. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Bishop J. M., Parker S., Westbrook K., Lewis G., Waite M. R. Na+ and K+ concentrations and the regulation of protein synthesis in Sindbis virus-infected chick cells. Virology. 1979 Jul 15;96(1):108–120. doi: 10.1016/0042-6822(79)90177-6. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Wright P. J., Cooper P. D. Poliovirus temperature-sensitivie mutants defective in cytopathic effects are also defective in synthesis of double-stranded RNA. J Gen Virol. 1975 Apr;27(1):45–59. doi: 10.1099/0022-1317-27-1-45. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Nair C. N., Stowers J. W., Singfield B. Guanidine-sensitive Na+ accumulation by poliovirus-infected HeLa cells. J Virol. 1979 Jul;31(1):184–189. doi: 10.1128/jvi.31.1.184-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Cleveland P. H., Shea M. A. Effect of mengovirus replication on choline metabolism and membrane formation in novikoff hepatoma cells. J Virol. 1970 Dec;6(6):800–812. doi: 10.1128/jvi.6.6.800-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G., Mannweiler K. Alterations of actin-containing structures in BHK21 cells infected with Newcastle disease virus and vesicular stomatitis virus. J Gen Virol. 1977 Nov;37(2):233–242. doi: 10.1099/0022-1317-37-2-233. [DOI] [PubMed] [Google Scholar]
- Sherline P., Schiavone K., Brocato S. Endogenous inhibitor of colchicine-tubulin binding in rat brain. Science. 1979 Aug 10;205(4406):593–595. doi: 10.1126/science.451622. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]


