Abstract
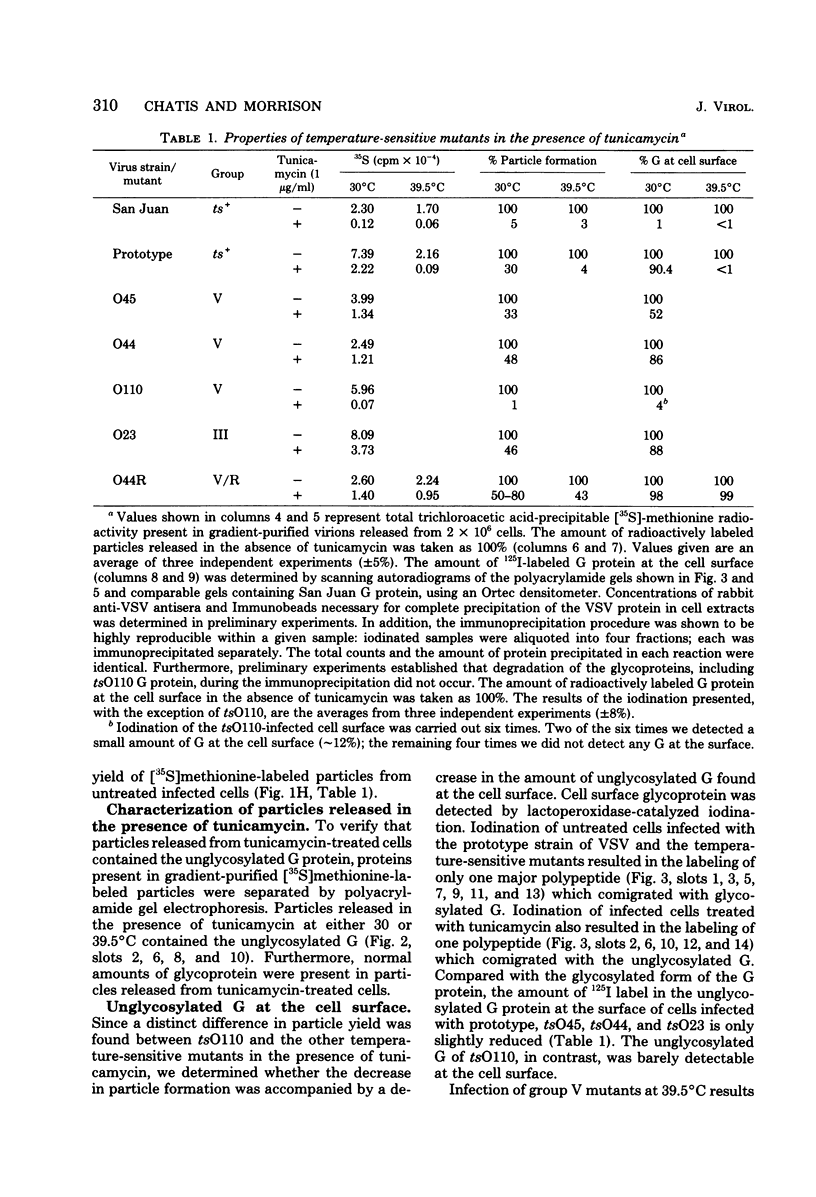
The role of carbohydrate in the morphogenesis of vesicular stomatitis virus was studied, using the antibiotic tunicamycin to inhibit glycosylation. It has been reported previously (Gibson et al., J. Biol. Chem. 254:3600-3607, 1979) that the San Juan strain of vesicular stomatitis virus requires carbohydrate for efficient migration of the glycoprotein (G) to the cell surface and for virion formation, whereas the prototype or Orsay strain of vesicular stomatitis virus is less stringent in its carbohydrate requirement at 30 degrees C. However, there are many differences between the two strains. We found that mutational changes within the G protein of the same strain of virus (prototype or Orsay) alters the requirement for carbohydrate at 30 degrees C. Group V or G protein mutants tsO45 and tsO44, like their prototype parent, did not require carbohydrate for efficient morphogenesis. In contrast, the G protein of another group V mutant, tsO110, was totally dependent upon carbohydrate addition for migration to the cell surface. Furthermore, no tsO110 particles were released in the absence of glycosylation. The wild-type prototype strain did require carbohydrate at 39.5 degrees C for insertion of the G protein into the plasma membrane and virion formation. However, a pseudorevertant of tsO44 (tsO44R), unlike the prototype parent, no longer exhibited this temperature-sensitive requirement for carbohydrate. At 39.5 degrees C in the presence of tunicamycin, tsO44R-infected cells released normal yields of particles and the unglycosylated G reached the cell surface very efficiently. In contrast to tsO110, which absolutely requires carbohydrate, mutational change in the tsO44R G protein has eliminated the requirement for carbohydrate. Thus, simple mutational changes, as opposed to many changes in the molecule, are sufficient to alter the carbohydrate requirement.
Full text
PDF

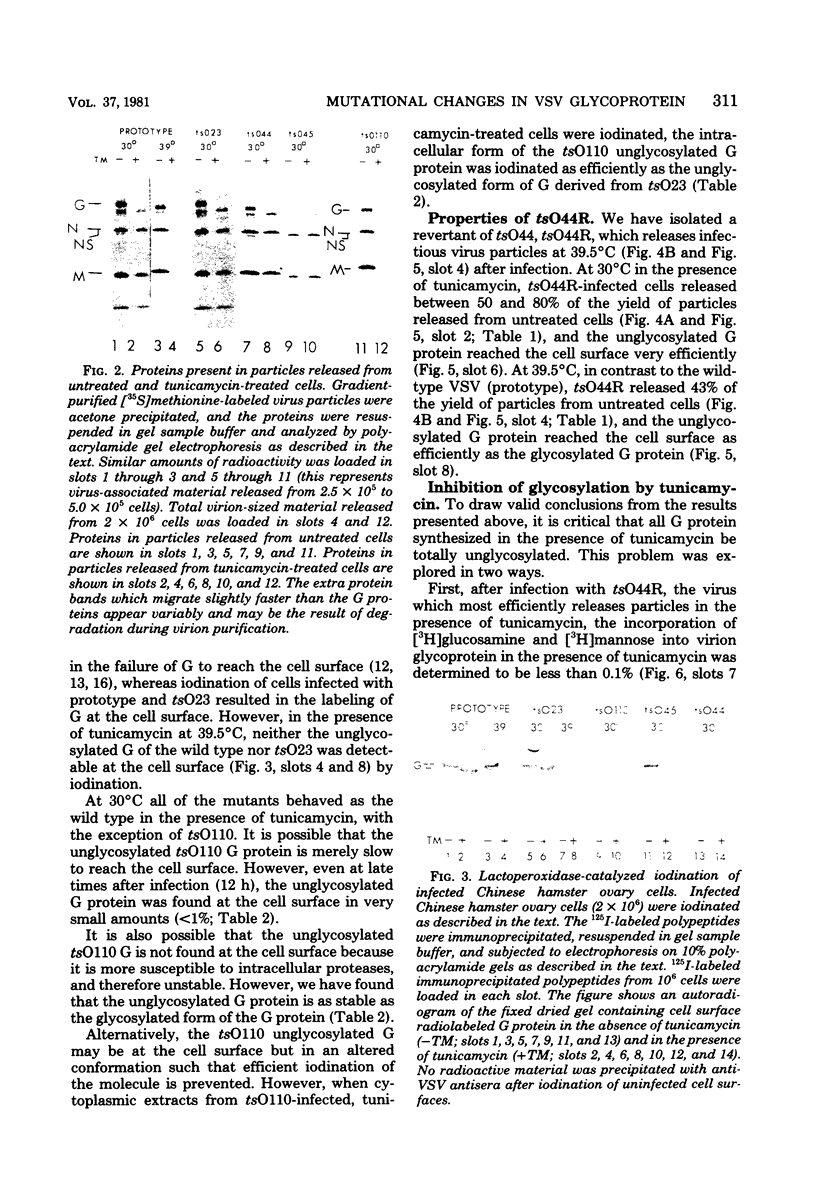
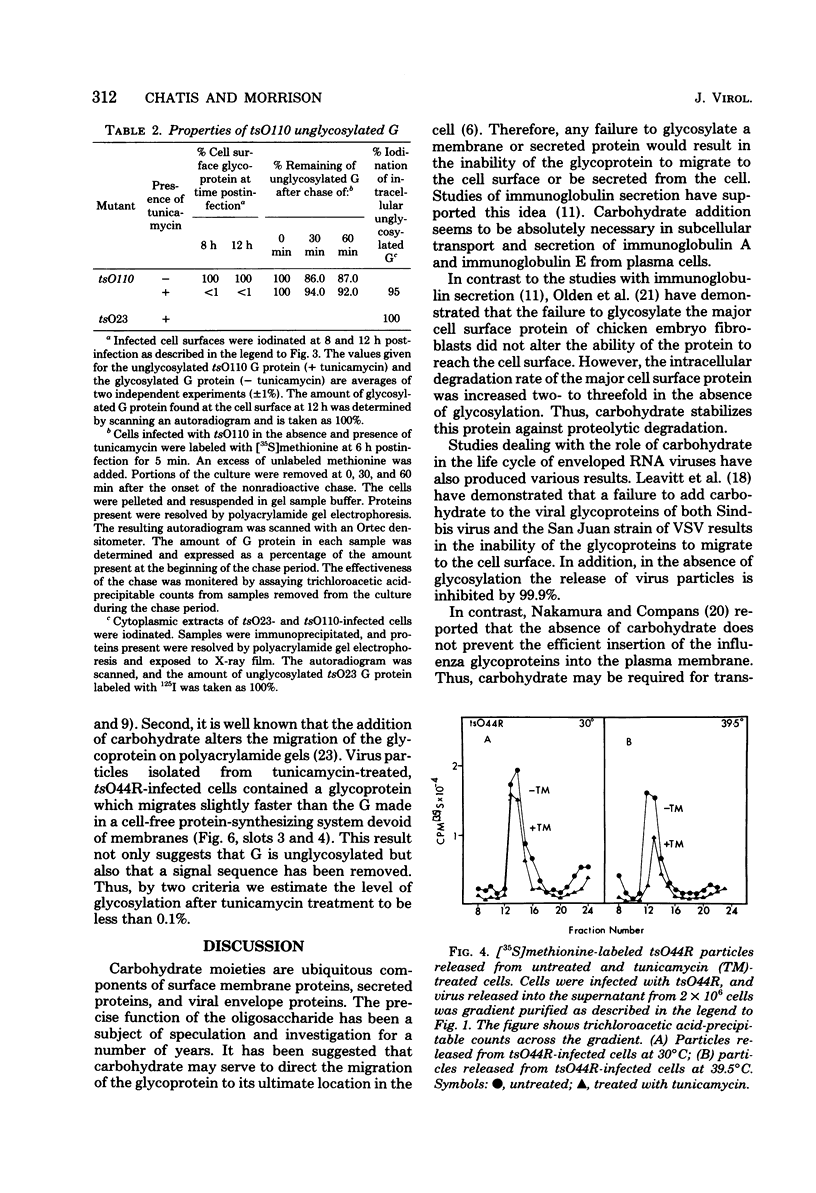


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Huang A. S. Conserved peptides in the proteins of vesicular stomatitis virus. Virology. 1979 Jun;95(2):445–453. doi: 10.1016/0042-6822(79)90499-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clinkscales C. W., Bratt M. A., Morrison T. G. Synthesis of Newcastle disease virus polypeptides in a wheat germ cell-free system. J Virol. 1977 Apr;22(1):97–101. doi: 10.1128/jvi.22.1.97-101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Rao D. D., Huang A. S. RNA synthesis of vesicular stomatitis virus. VIII. Oligonucleotides of the structural genes and mRNA. Gene. 1979 Feb;5(2):141–157. doi: 10.1016/0378-1119(79)90099-4. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F., Berkaloff A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire (VSV). Mutants de maturation. C R Acad Sci Hebd Seances Acad Sci D. 1969 Sep 15;269(11):1031–1034. [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Etude des mutants thermosensibles du Virus de la Stomatite Vésiculaire (VSV). Classification de quelques mutants d'après des critères de fonctionnement. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 12;268(19):2385–2388. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Newly made proteins zip through the cell. Science. 1980 Jan 11;207(4427):164–167. doi: 10.1126/science.7350651. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M., Sperling R. The cell-free synthesis and assembly of viral specific polypeptides into TMV particles. Virology. 1974 May;59(1):307–313. doi: 10.1016/0042-6822(74)90227-x. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]