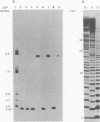
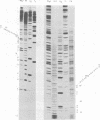
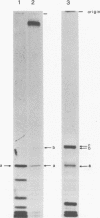
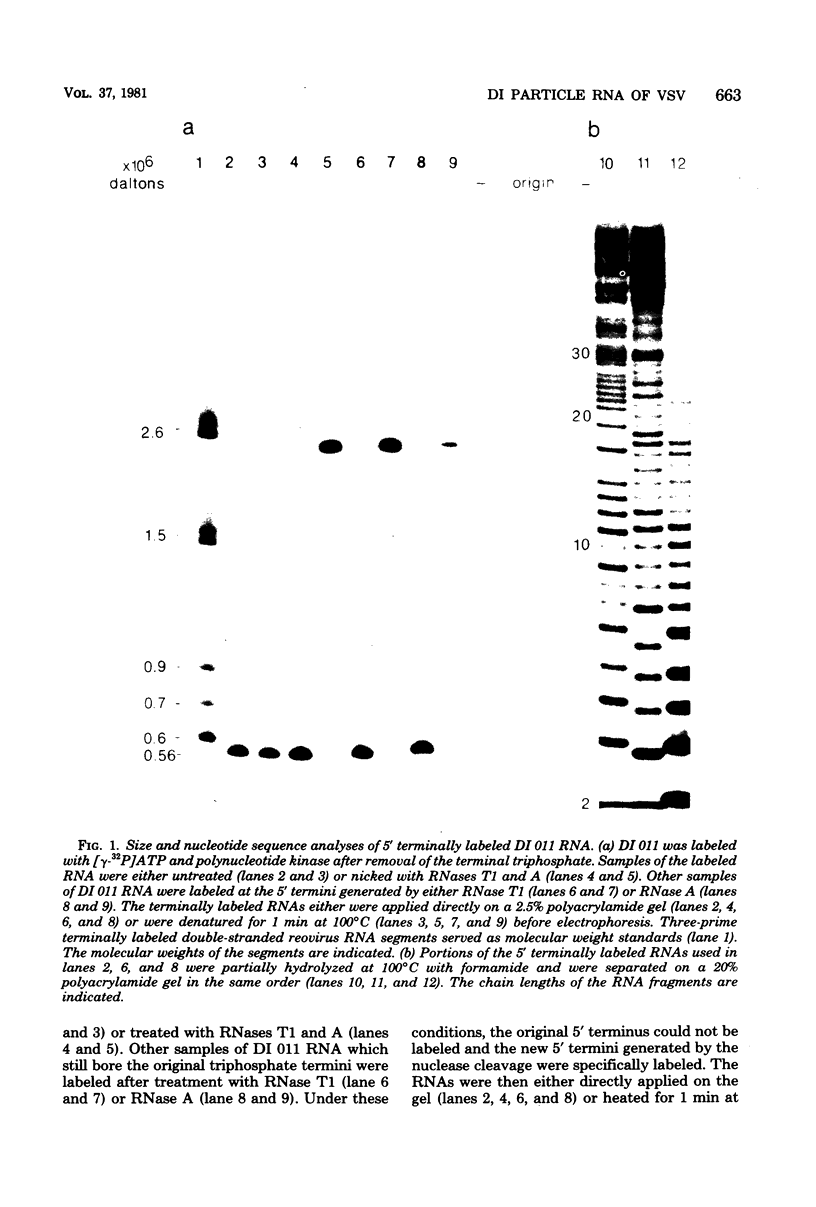
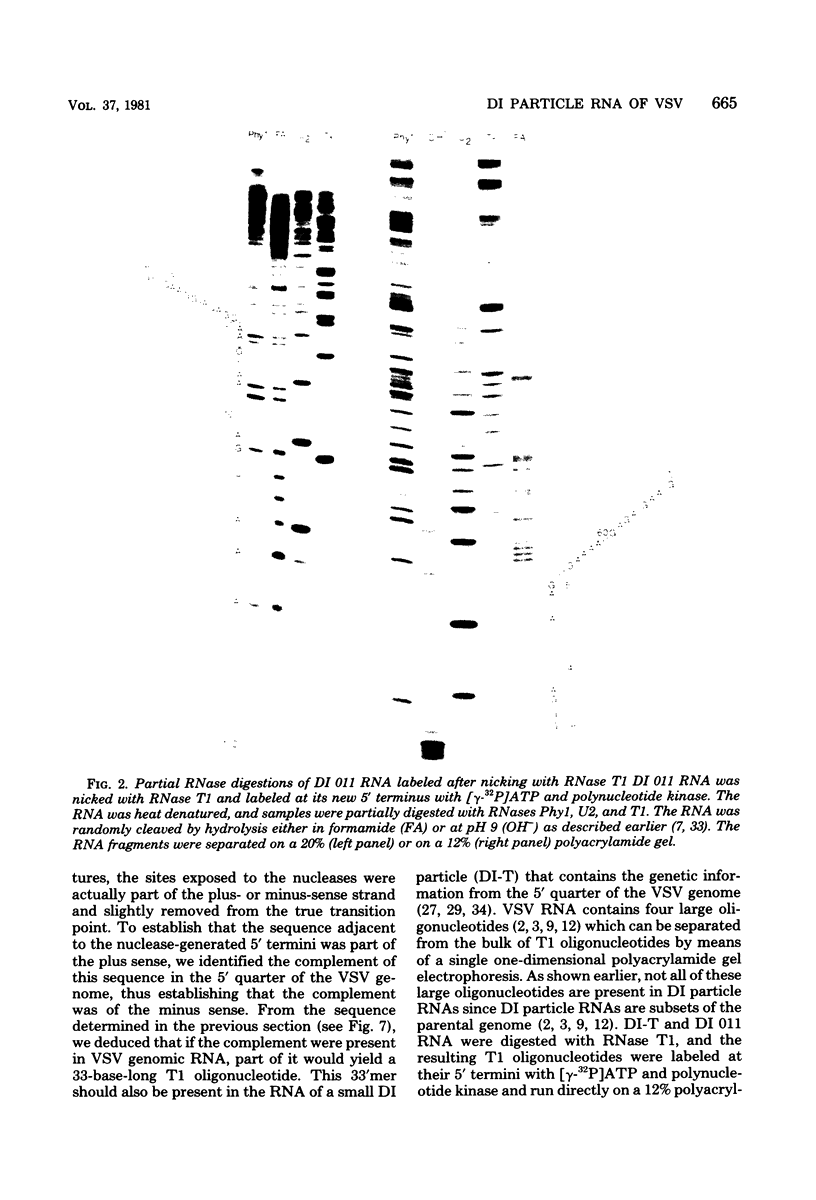
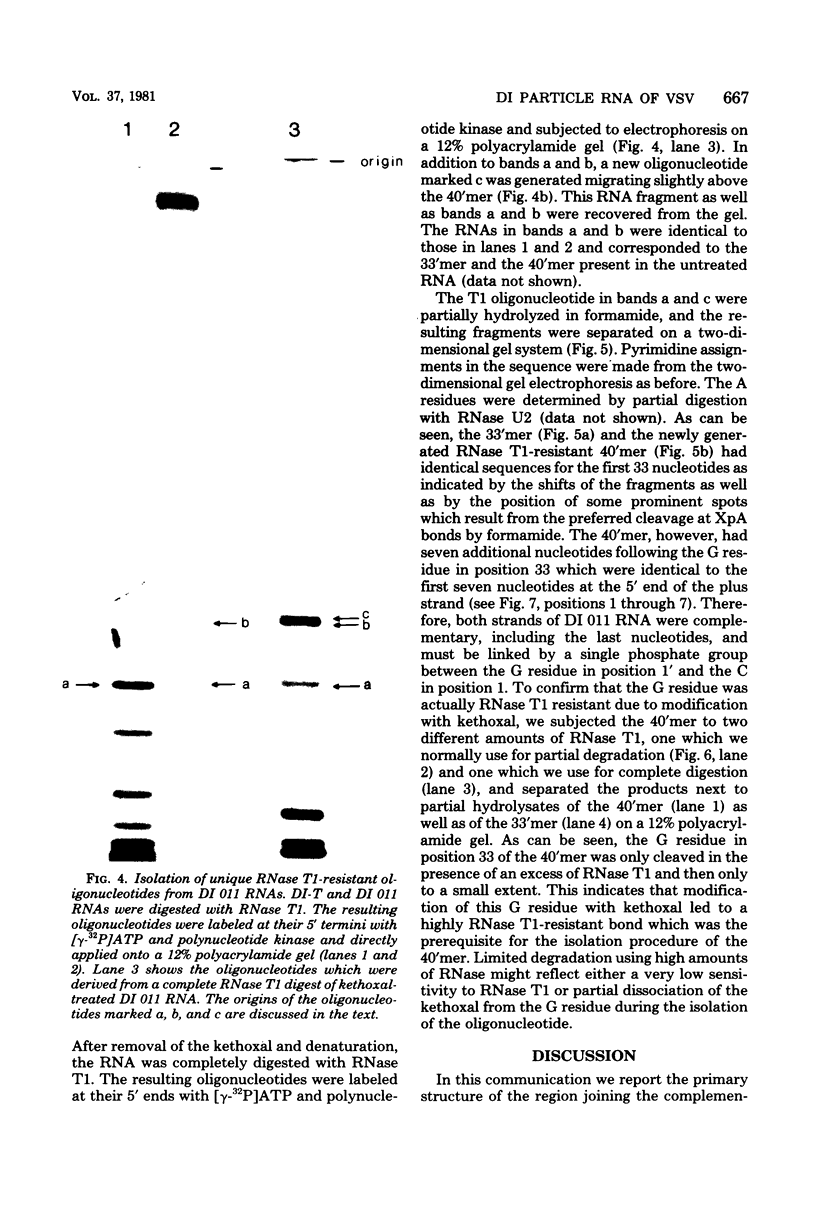
Abstract
The nucleotide sequence of the region which covalently links the complementary strands of the "snapback" RNA of vesicular stomatitis virus, DI011, is (Formula: see text). Both strands of the defective interfering (DI) particle RNA were complementary for their full length and were covalently linked by a single phosphate group. Because the strands were exactly the same length and complementary, template strand and daughter strand nucleocapsids generated during replication of DI 011 were undistinguishable on the basis of sequence, a property not shared by other types of DI particle RNAs. Treatment of the RNA with RNase T1 in high-ionic-strength solutions cleaved the RNA only between positions 1 and 1'. These results and the availability of the guanosine residue in position 1' to kethoxal, a reagent that specifically derivatizes guanosines of single-stranded RNA, suggest that steric constraints keep a small portion of the "turnaround" region in an open configuration. The sequence of the turnaround region was not related in any obvious way to the sequences at the 3' and 5' termini and limited the number of possible models for the origin of this type of DI particle RNA. Two models for the genesis of DI 011 RNA are discussed. We favor one in which the progenitor DI 011 RNA was generated by replication across a nascent replication fork.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy A. R., Joklik W. K. Studies on reovirus RNA. II. Characterization of reovirus messenger RNA and of the genome RNA segments from which it is transcribed. J Mol Biol. 1967 Oct 14;29(1):19–26. doi: 10.1016/0022-2836(67)90178-7. [DOI] [PubMed] [Google Scholar]
- Clerx-Van Haaster C. M., Clewley J. P., Bishop D. H. Oligonucleotide sequence analyses indicate that vesicular stomatitis virus large defective interfering virus particle RNA is made by internal deletion: evidence for similar transcription polyadenylation signals for the synthesis of all vesicular stomatitis virus mRNA species. J Virol. 1980 Feb;33(2):807–817. doi: 10.1128/jvi.33.2.807-817.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H. Assignment of the large oligonucleotides of vesicular stomatitis virus to the N, NS, M, G, and L genes and oligonucleotide gene ordering within the L gene. J Virol. 1979 Apr;30(1):116–123. doi: 10.1128/jvi.30.1.116-123.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Lazzarini R. A., Keene J. D., Banerjee A. K. In vitro synthesis of messenger RNA by a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 May;74(5):1884–1888. doi: 10.1073/pnas.74.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D. A., Herman R. C., Chien I., Lazzarini R. A. Defective interfering particle generated by internal deletion of the vesicular stomatitis virus genome. J Virol. 1980 Feb;33(2):818–829. doi: 10.1128/jvi.33.2.818-829.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of the 5' end of bacteriophage MS2 RNA Methoxyamine and kethoxal modification. Eur J Biochem. 1979 Dec 17;102(2):595–604. doi: 10.1111/j.1432-1033.1979.tb04277.x. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Lazzarini R. A. The 5' terminal nucleotide of RNA from vesicular stomatitis virus defective interfering particles. Virology. 1977 Apr;77(2):863–866. doi: 10.1016/0042-6822(77)90508-6. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Glimp T., Clewley J. P., Bishop D. H. Studies on the generation of vesicular stomatitis virus (indiana serotype) defective interfering particles. Virology. 1978 Jan;84(1):142–152. doi: 10.1016/0042-6822(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Litt M. Structural studies on transfer ribonucleic acid. I. Labeling of exposed guanine sites in yeast phenylalanine transfer ribonucleic acid with kethoxal. Biochemistry. 1969 Aug;8(8):3249–3253. doi: 10.1021/bi00836a017. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. F., Lazzarini R. A. Synthesis of viral mRNA and polyadenylate by a ribonucleoprotein complex from extracts of VSV-infected cells. Cell. 1974 Sep;3(1):77–84. doi: 10.1016/0092-8674(74)90043-9. [DOI] [PubMed] [Google Scholar]
- Perrault J. Cross-linked double-stranded RNA from a defective vesicular stomatitis virus particle. Virology. 1976 Apr;70(2):360–371. doi: 10.1016/0042-6822(76)90278-6. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petric M., Prevec L. Vesicular stomatitis virus--a new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970 Aug;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. The size and the cistronic origin of defective vesicular stomatitis virus particle RNAs in relation to homotypic and heterotypic interference. J Mol Biol. 1976 Mar 5;101(3):307–325. doi: 10.1016/0022-2836(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Holland J. J. The nucleotide sequence of the 5' terminus of vesicular stomatitis virus RNA. Nucleic Acids Res. 1979 Aug 24;6(12):3923–3931. doi: 10.1093/nar/6.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]