Abstract
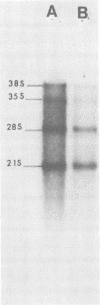
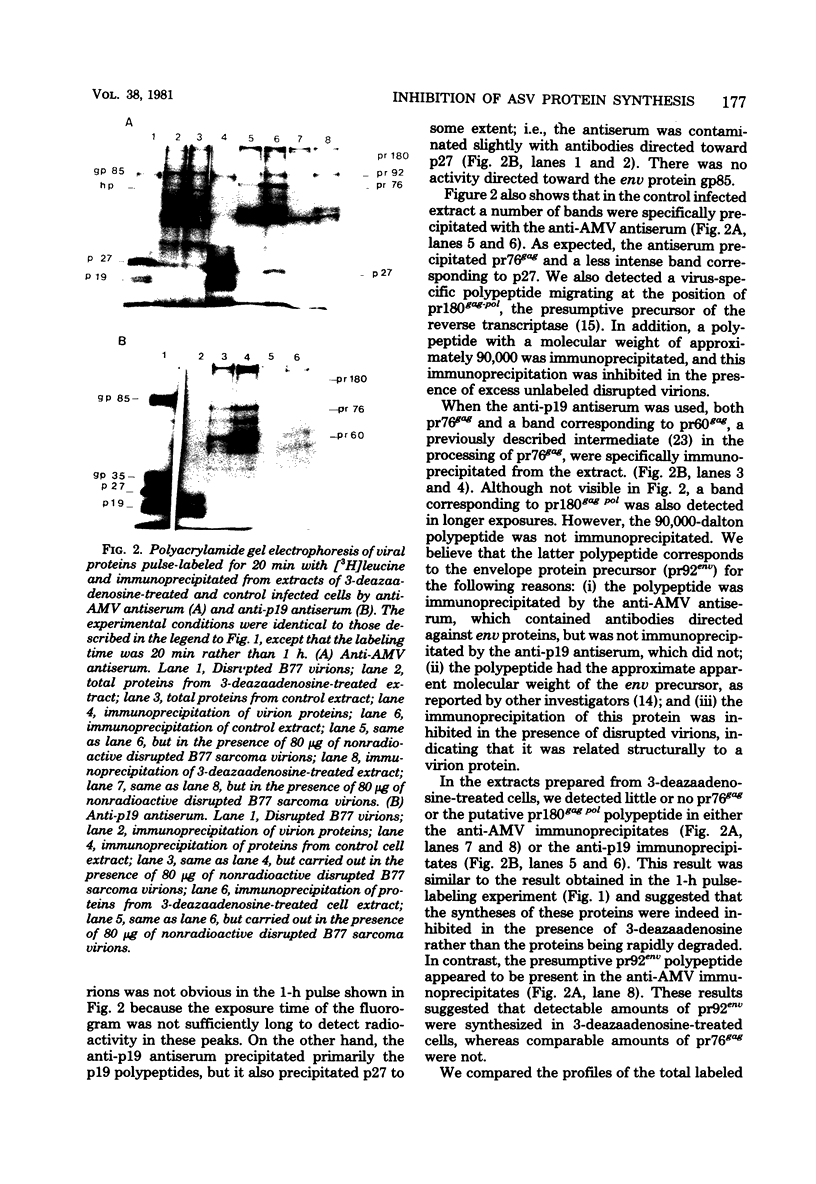
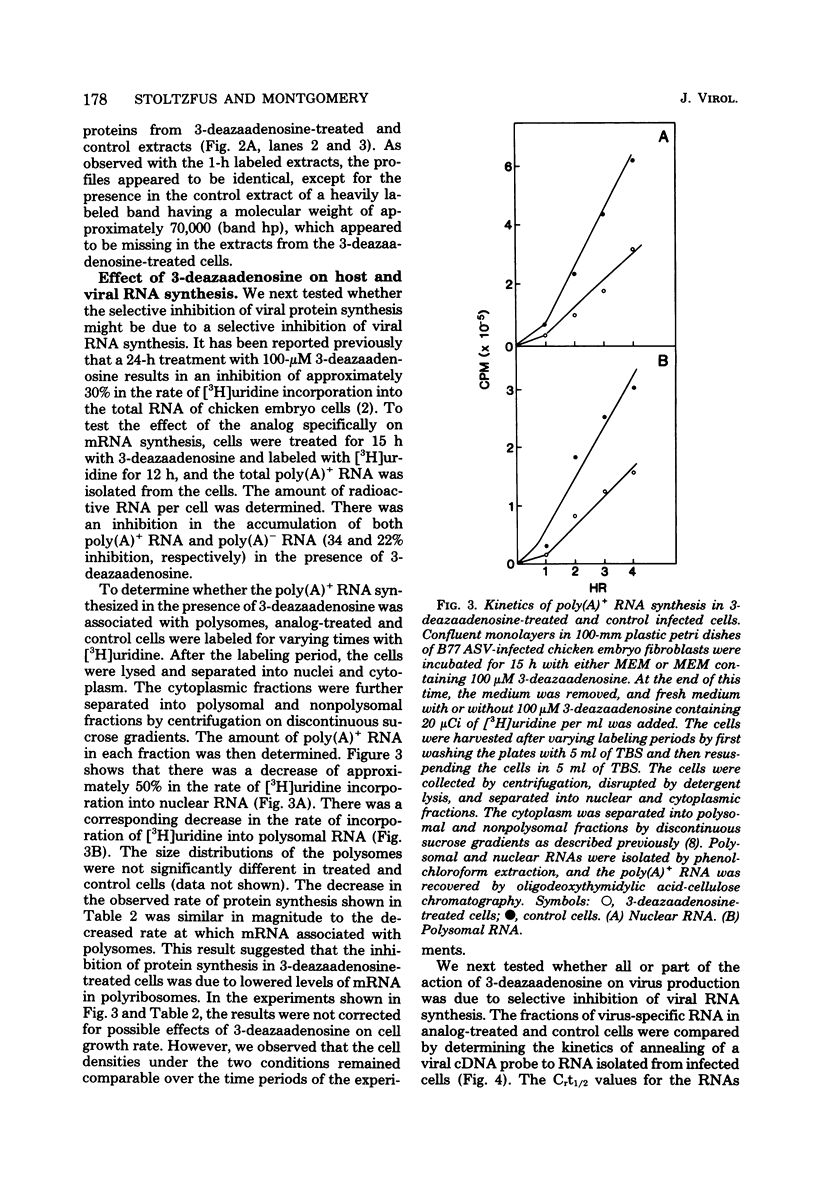
The production of B77 avian sarcoma virions was inhibited more than 90% in infected chicken embryo fibroblasts that were treated with 100 microM 3-deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase and, for this reason, an inhibitor of methylation reactions. This nucleoside analog at a concentration of 100 microM inhibited the rates of overall cellular protein synthesis and polyadenylated RNA synthesis by 40 to 50%. Rates of viral protein synthesis were compared, and the results indicated that in infected cells treated with 3-deazaadenosine syntheses of both the precursor of the gag proteins (pr76gag) and the precursor of the reverse transcriptase (pr180gag pol) were inhibited. Synthesis of the precursor of the viral envelope glycoproteins (pr92env) appeared to be affected less by the analog treatment. Most of the host polypeptides also continued to be synthesized in 3-deazaadenosine-treated cells. The fraction of the total RNA represented by virus-specific RNA in the 3-deazaadenosine-treated cells was approximately 40% of the fraction of the total RNA represented by viral RNA in control cells, as determined by hybridization kinetics. Therefore, there was a selective inhibition of viral RNA accumulation in the presence of 3-deazaadenosine. The amounts of genome-sized 35S and 38S RNAs were reduced compared with the amounts of 28S and 21S viral mRNA's. These results suggest that selective inhibition of the synthesis of viral proteins is due to selective decreases in the amounts of the mRNA's for these polypeptides.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R., Chiang P. K., Cantoni G. L. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978 Sep;89(2):494–505. doi: 10.1016/0042-6822(78)90191-5. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Processing and function of undermethylated chicken embryo fibroblast mRNA. J Biol Chem. 1979 Jul 10;254(13):5591–5594. [PubMed] [Google Scholar]
- Dimock K., Stolzfus C. M. Cycloleucine blocks 5'-terminal and internal methylations of avian sarcoma virus genome RNA. Biochemistry. 1978 Aug 22;17(17):3627–3632. doi: 10.1021/bi00610a032. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Kuhnert L. K. Evidence for the identity of shared 5'-terminal sequences between genome RNA and subgenomic mRNA's of B77 avian sarcoma virus. J Virol. 1979 Nov;32(2):536–545. doi: 10.1128/jvi.32.2.536-545.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]