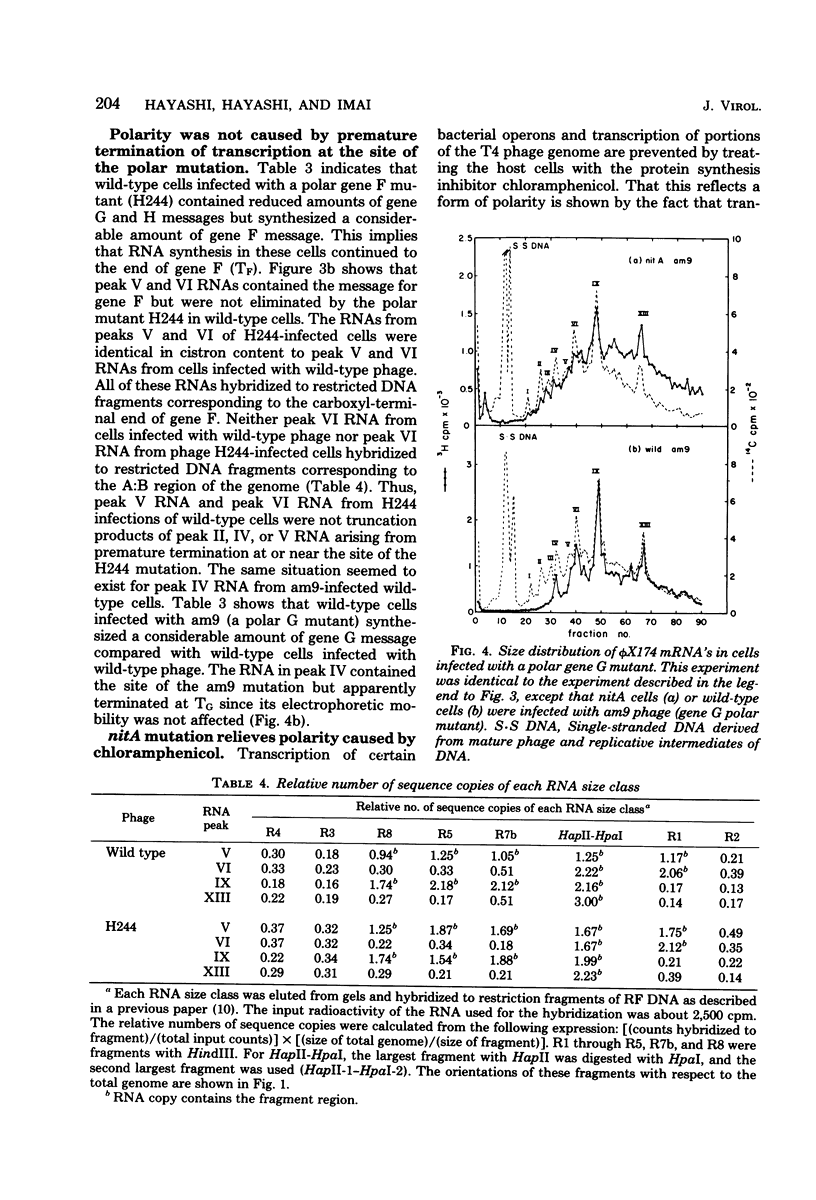
Abstract
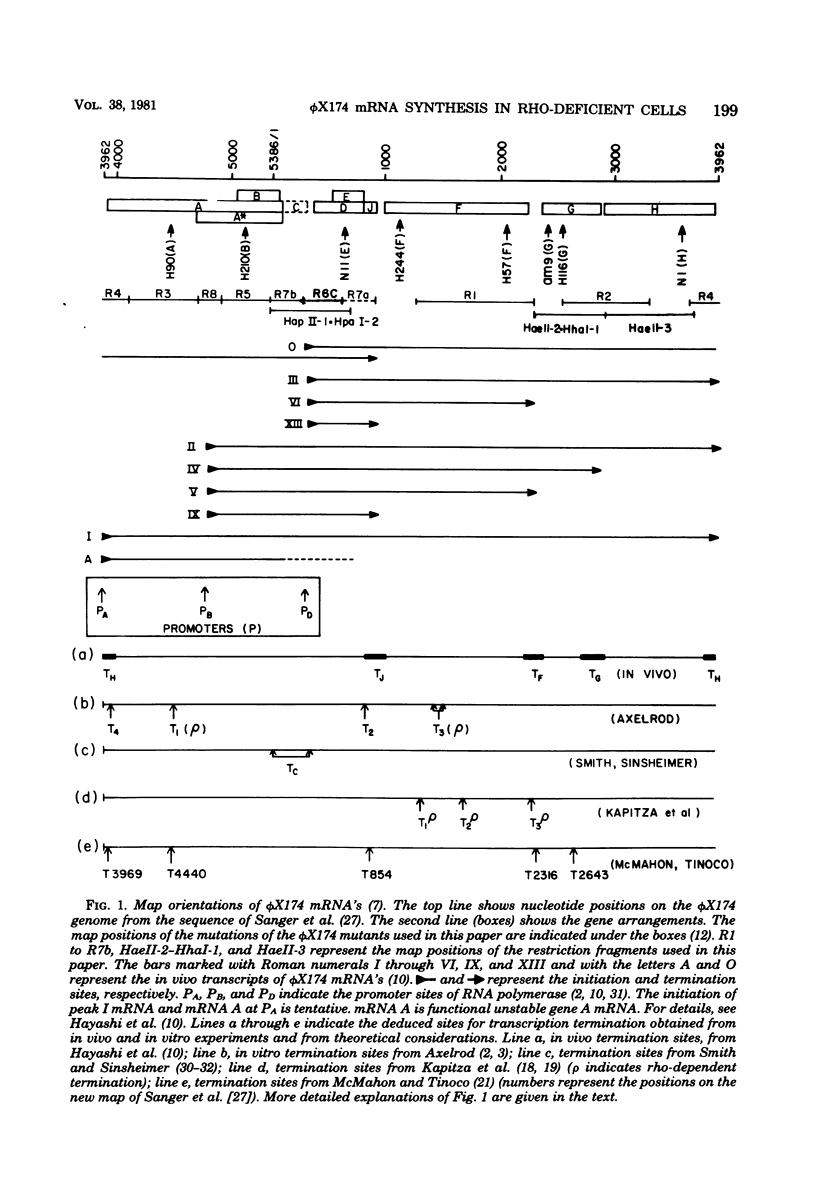
A previous report (Hayashi et al., Proc. Natl. Acad. Sci. U.S.A. 73:3519-3523, 1976) indicated that in vivo bacteriophage phi X174 mRNA's terminate after genes J, F, G, and H. However, termination at these sites is not stringent. To determine whether termination of phi X174 transcription depends on rho factor activity, we introduced a temperature-sensitive rho mutation (nitA) into a phi X174-sensitive host cell line and determined termination sites in wild-type and nitA cells. We found that (i) normal phi X174 terminators were recognized in phi X174-infected nitA cells, (ii) the rho mutation relieved polar effects caused by nonsense mutations in the phage genome or by chloramphenicol treatment of the host cells, and (iii) polarity was not caused by premature termination of transcription at the site of the polar mutation. RNA synthesis continued beyond the site to the first rho-sensitive site.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Transcription of bacteriophage phi-X174 in vitro: selective initiation with oligonucleotides. J Mol Biol. 1976 Dec 25;108(4):753–770. doi: 10.1016/s0022-2836(76)80115-5. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Transcription of bacteriophage phiX174 in vitro: analysis with restriction enzymes. J Mol Biol. 1976 Dec 25;108(4):771–779. doi: 10.1016/s0022-2836(76)80116-7. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXVII. RNA metabolism in phiX174-infected cells. J Virol. 1975 Jan;15(1):151–160. doi: 10.1128/jvi.15.1.151-160.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Hayashi M. Electrophoretic characterization of phiX174-specific proteins. J Mol Biol. 1969 Sep 28;44(3):501–516. doi: 10.1016/0022-2836(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Fragment maps of phiX-174 replicative DNA produced by restriction enzymes from haemophilus aphirophilus and haemophilus influenzae H-I. J Virol. 1974 Nov;14(5):1142–1151. doi: 10.1128/jvi.14.5.1142-1151.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Isolation of phi X174 specific messenger ribonucleic acids in vivo and identification of their 5' terminal nucleotides. J Virol. 1972 Feb;9(2):207–215. doi: 10.1128/jvi.9.2.207-215.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Fujimura F. K., Hayashi M. Mapping of in vivo messenger RNAs for bacteriophage phiX-174. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3519–3523. doi: 10.1073/pnas.73.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Shigesada K. Studies on the altered rho factor in a nitA mutants of Escherichia coli defective in transcription termination. I. Characterization and quantitative determination of rho in cell extracts. J Mol Biol. 1978 Apr 25;120(4):451–466. doi: 10.1016/0022-2836(78)90348-0. [DOI] [PubMed] [Google Scholar]
- Inoko H., Imai M. Isolation and genetic characterization of the nitA mutants of Escherichia coli affecting the termination factor rho. Mol Gen Genet. 1976 Jan 16;143(2):211–221. doi: 10.1007/BF00266924. [DOI] [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng Y., Gelfand D., Hayashi M., Shleser R., Tessman E. S. The eight genes of bacteriophages phi X174 and S13 and comparison of the phage-specified proteins. J Mol Biol. 1970 Apr 28;49(2):521–526. doi: 10.1016/0022-2836(70)90262-7. [DOI] [PubMed] [Google Scholar]
- Kapitza E. L., Stuckacheva E. A., Shemyakin M. F. The effect of the termination rho factor and ribonuclease III on the transcription of bacteriophage phi X174 DNA in vitro. FEBS Lett. 1976 Apr 15;64(1):81–84. doi: 10.1016/0014-5793(76)80254-2. [DOI] [PubMed] [Google Scholar]
- Kapitza E. L., Stukacheva E. A., Shemyakin M. F. Effect of Escherichia coli rho factor and RNase III on the formation of phi X174 RNA in vitro. FEBS Lett. 1979 Feb 1;98(1):123–127. doi: 10.1016/0014-5793(79)80167-2. [DOI] [PubMed] [Google Scholar]
- McMahon J. E., Tinoco I., Jr Sequences and efficiencies of proposed mRNA terminators. Nature. 1978 Jan 19;271(5642):275–277. doi: 10.1038/271275a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Shigesada K., Imai M. Studies on the altered rho factor in nitA mutants of Escherichia coli defective in transcription termination. II. Purification and molecular properties of the mutant rho. J Mol Biol. 1978 Apr 25;120(4):467–486. doi: 10.1016/0022-2836(78)90349-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Gottesman M., Tomczak K., Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. H., Sinsheimer R. L. The in vitro transcription units of bacteriophage phiX174. I. Characterization of synthetic parameters and measurement of transcript molecular weights. J Mol Biol. 1976 Jun 5;103(4):681–697. doi: 10.1016/0022-2836(76)90203-5. [DOI] [PubMed] [Google Scholar]
- Smith L. H., Sinsheimer R. L. The in vitro transcription units of bacteriophage phiX174. II. In vitro initiation sites of phiX174 transcription. J Mol Biol. 1976 Jun 5;103(4):699–710. doi: 10.1016/0022-2836(76)90204-7. [DOI] [PubMed] [Google Scholar]
- Smith L. H., Sinsheimer R. L. The in vitro transcription units of bacteriophage phiX174. III. Initiation with specific 5' end oligonucleotides of in vitro phiX174 RNA. J Mol Biol. 1976 Jun 5;103(4):711–735. doi: 10.1016/0022-2836(76)90205-9. [DOI] [PubMed] [Google Scholar]
- Vanderbilt A. S., Borrás M. T., Germeraad S., Tessman I., Tessman E. S. A promoter site and polarity gradients in phage S13. Virology. 1972 Oct;50(1):171–179. doi: 10.1016/0042-6822(72)90357-1. [DOI] [PubMed] [Google Scholar]
- Vanderbilt A. S., Borrás M. T., Tessman E. S. Direction of translation in phage S13 as determined from the sizes of polypeptide fragments of nonsense mutants. Virology. 1971 Feb;43(2):352–355. doi: 10.1016/0042-6822(71)90307-2. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]


