Abstract
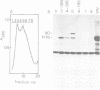
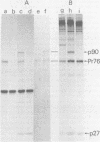
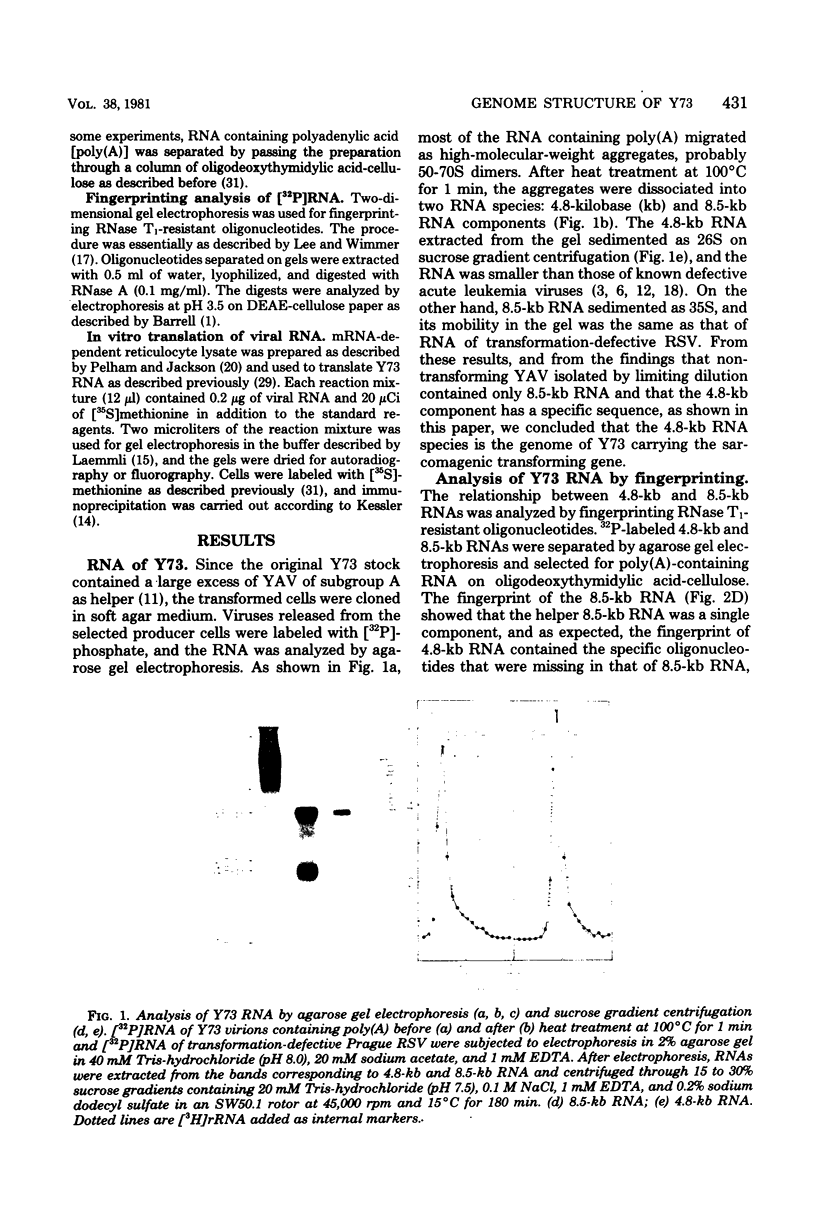
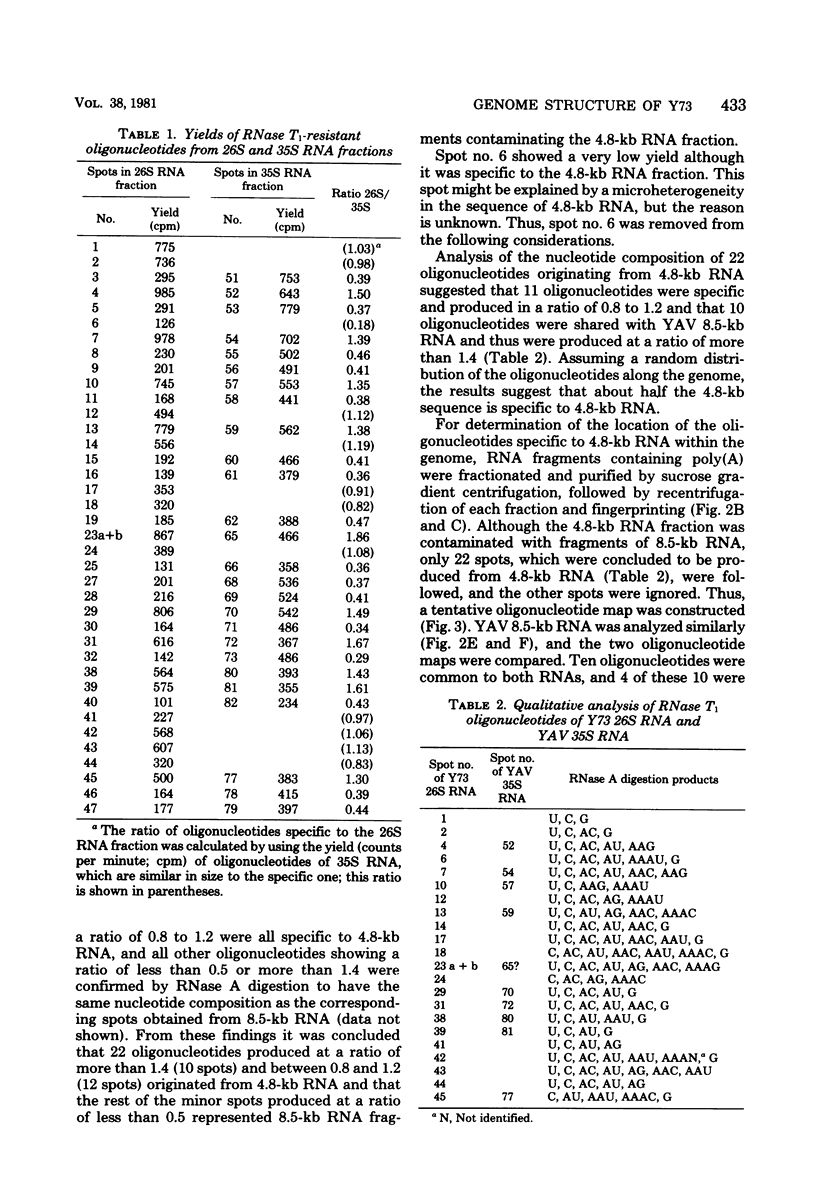
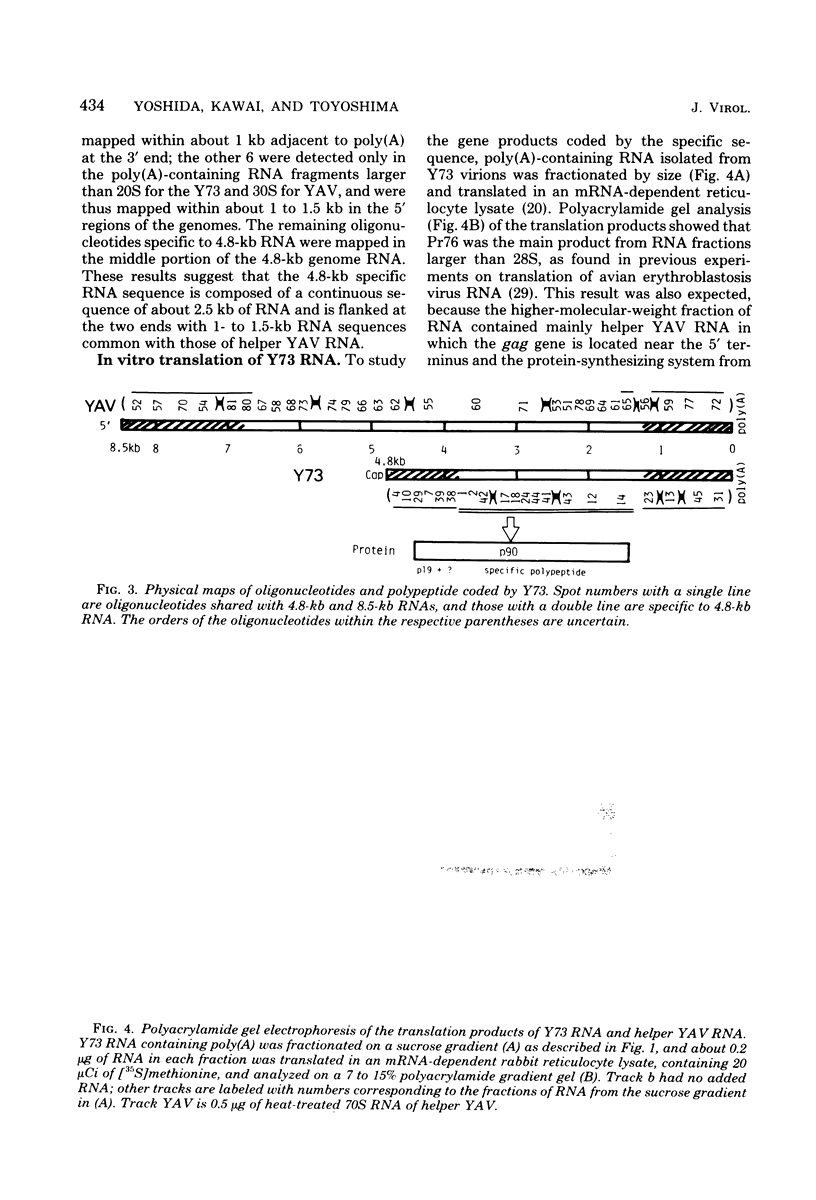
The genome structure of a newly isolated sarcoma virus, Y73, was studied. Y73 is a defective, potent sarcomagenic virus and contains 4.8-kilobase (kb) RNA as its genome; in contrast, helper virus associated with Y73 had 8.5-kb RNA, similar to other avian leukemia viruses. Fingerprinting analysis these RNAs demonstrated that the 4.8-kb RNA contains a specific RNA sequence of 2.5 kb, which represents the transforming gene (yas) of Y73. This specific sequence was mapped in the middle of the genome and had at both ends 1- to 1.5-kb sequences in common with Y73-associated virus RNA. This structure is very similar to those of avian and mammalian leukemia viruses. In vitro translation of the 4.8-kb RNA and the immunospecificity of the products directly demonstrated that polyprotein p90, containing p19, is a product translated from capped 4.8-kb RNA and that the specific peptide portion is coded by the yas sequence. Protein 90, which was also found in cells transformed with Y73, was suggested to be a transforming protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Hirata K., Inoue M., Hatsuoka M., Sato A. Isolation of a sarcoma virus from a spontaneous chicken tumor. Gan. 1978 Dec;69(6):825–830. [PubMed] [Google Scholar]
- Kamahora T., Sugiyama H., Nomoto A., Yoshida M., Toyshima K. RNA specific for the transforming component of avian erythroblastosis virus strain R. Virology. 1979 Jul 15;96(1):291–294. doi: 10.1016/0042-6822(79)90196-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Goff S., Paskind M., Otto G., Baltimore D. Structure of the Abelson murine leukemia virus genome. Cell. 1979 Dec;18(4):955–962. doi: 10.1016/0092-8674(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Strain specificity of changes in adenylate cyclase activity in cells transformed by avian sarcoma viruses. Virology. 1975 Jan;63(1):68–76. doi: 10.1016/0042-6822(75)90371-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Toyoshima K. In vitro translation of avian erythroblastosis virus RNA: identification of two major polypeptides. Virology. 1980 Jan 30;100(2):484–487. doi: 10.1016/0042-6822(80)90538-3. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Yamashita M., Nomoto A. Transformation-defective mutants of Rous sarcoma virus with longer sizes of genome RNA and their highly frequent occurrences. J Virol. 1979 May;30(2):453–461. doi: 10.1128/jvi.30.2.453-461.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Yoshikura H. Analysis of spleen focus-forming virus-specific RNA sequences coding for spleen focus-forming virus-specific glycoprotein with a molecular weight of 55,000 (gp55). J Virol. 1980 Feb;33(2):587–596. doi: 10.1128/jvi.33.2.587-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]