Abstract
A lipopolysaccharide (LPS)-defective (rough) mutant of Pseudomonas aeruginosa PAO was isolated by selection for resistance to the LPS-specific phage E79. The LPS of this mutant, AK-1012, lacked the O-antigenic side chain-specific amino sugar fucosamine as well as the core-specific sugars glucose and rhamnose. Using this strain, we isolated and characterized a phage, phi PLS27, which is specifically inactivated upon incubation with LPS extracted from rough mutants of P. aeruginosa PAO. phi PLS27 was found to be a Bradley type C phage and was very similar to coliphage T7 in a number of properties, including size, buoyant density, mass, and the number of structural proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Friedmann A. Practical procedures for the purification of bacterial viruses. Appl Microbiol. 1971 Oct;22(4):706–715. doi: 10.1128/am.22.4.706-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Bowes J. M., Dowell C. E. Purification and some properties of bacteriophage ST-1. J Virol. 1974 Jan;13(1):53–61. doi: 10.1128/jvi.13.1.53-61.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- CSAKY T. Z., BEARD D., DILLON E. S., BEARD J. W. Chemical analysis of the T7 bacteriophage of Escherichia coli. J Biol Chem. 1950 Jul;185(1):311–321. [PubMed] [Google Scholar]
- Chester I. R., Gray G. W., Wilkinson S. G. Further studies of the chemical composition of the lipopolysaccharide of Pseudomonas aeruginosa. Biochem J. 1972 Jan;126(2):395–407. doi: 10.1042/bj1260395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of T7 bacteriophage. J Mol Biol. 1962 Dec;5:635–642. doi: 10.1016/s0022-2836(62)80091-6. [DOI] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Symes K. C., Gray G. W., Wilkinson S. G. Studies of polysaccharide fractions from the lipopolysaccharide of Pseudomonas aeruginosa N.C.T.C. 1999. Biochem J. 1975 Jul;149(1):93–106. doi: 10.1042/bj1490093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Feige U., Stirm S. On the structure of the Escherichia coli C cell wall lipopolysaccharide core and on its phiX174 receptor region. Biochem Biophys Res Commun. 1976 Jul 26;71(2):566–573. doi: 10.1016/0006-291x(76)90824-x. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Meadow P. M. Evidence for two regions in the polysaccharide moiety of the lipopolysaccharide of Pseudomonas aeruginosa 8602. FEBS Lett. 1970 Jul 29;9(2):81–84. doi: 10.1016/0014-5793(70)80318-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gormus B. J., Wheat R. W. Polysaccharides of type 6 Klebsiella. J Bacteriol. 1971 Dec;108(3):1304–1309. doi: 10.1128/jb.108.3.1304-1309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: production of contractile and flexuous pyocins in Pseudomonas aeruginosa. J Gen Microbiol. 1974 Jan;80(1):17–30. doi: 10.1099/00221287-80-1-17. [DOI] [PubMed] [Google Scholar]
- Hannecart-Pokorni E., Godard C., Beumer J. Chimiotypes de mutants R de Shigella flexneri et récepteurs de phages. II. -- Localisation des récepteurs de phages. Ann Microbiol (Paris) 1976 Jul;127(1):15–24. [PubMed] [Google Scholar]
- Ikeda K., Egami F. Receptor substance for pyocin R. I. Partial purification and chemical properties. J Biochem. 1969 Apr;65(4):603–609. doi: 10.1093/oxfordjournals.jbchem.a129053. [DOI] [PubMed] [Google Scholar]
- Jarrell K., Kropinski A. M. Identification of the cell wall receptor for bacteriophage E79 in Pseudomonas aeruginosa strain PAO. J Virol. 1977 Sep;23(3):461–466. doi: 10.1128/jvi.23.3.461-466.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
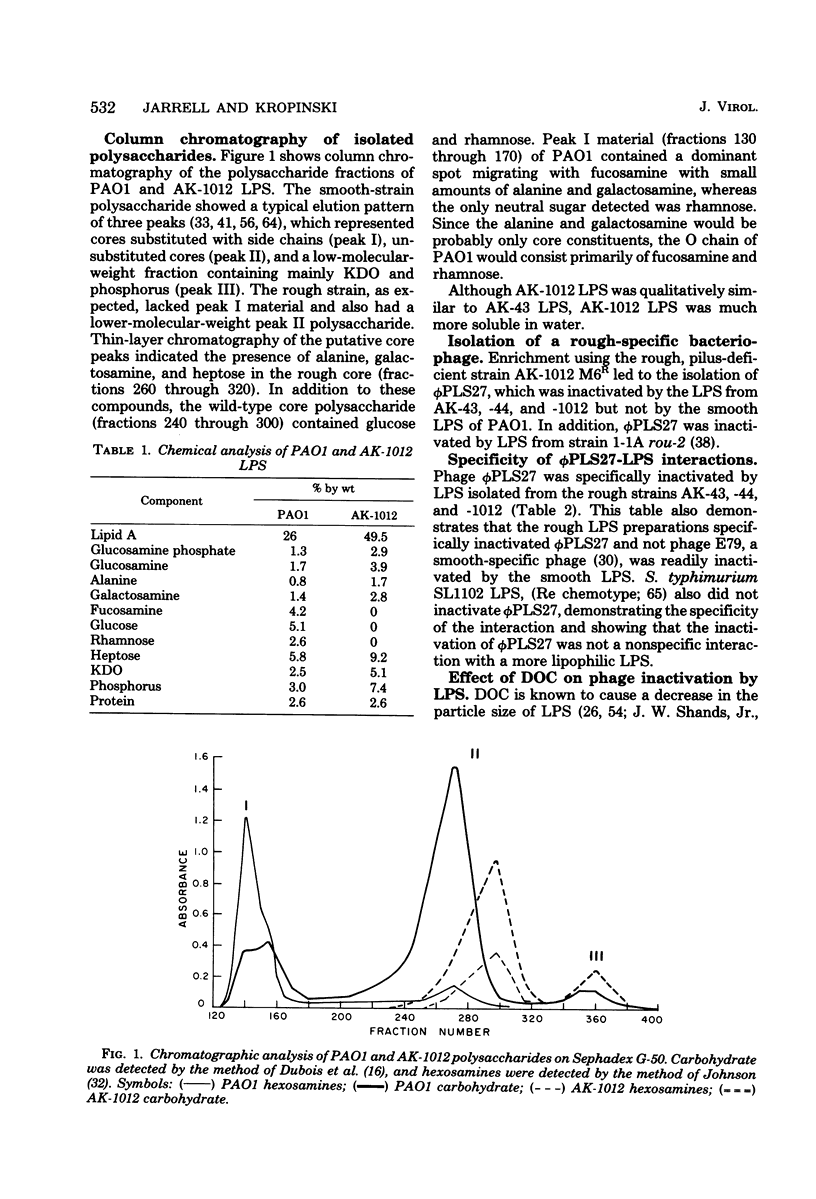
- Jarrell K., Kropinski A. M. The chemical composition of the lipopolysaccharide from Pseudomonas aeruginosa strain PAO and a spontaneously derived rough mutant. Microbios. 1977;19(76):103–116. [PubMed] [Google Scholar]
- Jarrell K., Kropinski A. M. The isolation and characterization of a lipopolysaccharide-specific Pseudomonas aeruginosa bacteriophage. J Gen Virol. 1976 Oct;33(1):99–106. doi: 10.1099/0022-1317-33-1-99. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The lipopolysaccharide receptor for bacteriophage phiX174 and S13. Virology. 1975 Jul;66(1):268–282. doi: 10.1016/0042-6822(75)90197-x. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Improved method of hexosamine determination. Anal Biochem. 1971 Dec;44(2):628–635. doi: 10.1016/0003-2697(71)90252-1. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B., McDonald I. J. Studies of the cellular and free lipopolysaccharides form Neisseria canis and N. subflava. Can J Microbiol. 1976 Feb;22(2):189–196. doi: 10.1139/m76-026. [DOI] [PubMed] [Google Scholar]
- Kelln R. A., Warren R. A. Isolation and properties of a bacteriophage lytic for a wide range of pseudomonads. Can J Microbiol. 1971 May;17(5):677–682. doi: 10.1139/m71-109. [DOI] [PubMed] [Google Scholar]
- Key B. A., Gray G. W., Wilkinson S. G. The purification and chemical composition of the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1970 Dec;120(3):559–566. doi: 10.1042/bj1200559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Botstein D., Casjens S., Earnshaw W., Harrison S., Lenk E. Structure and assembly of the capsid of bacteriophage P22. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):37–49. doi: 10.1098/rstb.1976.0096. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Meadow P. M. The isolation and characterization of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa PAC1. J Gen Microbiol. 1977 Feb;98(2):387–398. doi: 10.1099/00221287-98-2-387. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chadwick J. S. The pathogenicity of rough strains of Pseudomonas aeruginosa for Galleria mellonella. Can J Microbiol. 1975 Dec;21(12):2084–2088. doi: 10.1139/m75-297. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L. C., Milazzo F. H. The extraction and analysis of lipopolysaccharides from Pseudomonas aeruginosa strain PAO, and three rough mutants. Can J Microbiol. 1979 Mar;25(3):390–398. doi: 10.1139/m79-060. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Jarrell K., Milazzo F. H. The nature of Pseudomonas aeruginosa strain PAO bacteriophage receptors. Can J Microbiol. 1977 Jun;23(6):653–658. doi: 10.1139/m77-098. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Influence of O side chains on the attachment of the Felix O-1 bacteriophage to Salmonella bacteria. J Bacteriol. 1969 Aug;99(2):513–519. doi: 10.1128/jb.99.2.513-519.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Studies of a receptor for felix O-1 phage in Salmonella minnesota. J Gen Microbiol. 1967 Aug;48(2):225–233. doi: 10.1099/00221287-48-2-225. [DOI] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Comparison of blue-green algae virus LPP-1 and the morphologically related viruses G-3 and coliphage T7. Virology. 1968 Apr;34(4):675–678. doi: 10.1016/0042-6822(68)90088-3. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M. F116: a DNA bacteriophage specific for the pili of Pseudomonas aeruginosa strain PAO. Virology. 1973 Oct;55(2):558–560. doi: 10.1016/0042-6822(73)90203-1. [DOI] [PubMed] [Google Scholar]
- Phillips I. Identification of Pseudomonas aeruginosa in the clinical laboratory. J Med Microbiol. 1969 Feb;2(1):9–16. doi: 10.1099/00222615-2-1-9. [DOI] [PubMed] [Google Scholar]
- Quirk A. V., Sletten A., Hignett R. C. Properties of phage-receptor lipopolysaccharide from Pseudomonas morsprunorum. J Gen Microbiol. 1976 Oct;96(2):375–381. doi: 10.1099/00221287-96-2-375. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. A., Kropinski A. M. Separation of amino sugars and related compounds by two-dimensional thin-layer chromatography. J Chromatogr. 1980 Jul 4;195(1):127–132. doi: 10.1016/s0021-9673(00)81550-2. [DOI] [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Takeda K., Uetake H. In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage epsilon15. Virology. 1973 Mar;52(1):148–159. [PubMed] [Google Scholar]
- Wiener S., Urivetzky M., Lendvai S., Shafer S., Meilman E. The indole method for determination of DNA: conditions for maximal sensitivity. Anal Biochem. 1976 Apr;71(2):579–582. doi: 10.1016/s0003-2697(76)80027-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zajac E., Russa R., Lorkiewicz Z. Lipopolysaccharide as receptor for rhizobium phage 1P. J Gen Microbiol. 1975 Oct;90(2):365–367. doi: 10.1099/00221287-90-2-365. [DOI] [PubMed] [Google Scholar]




