Abstract
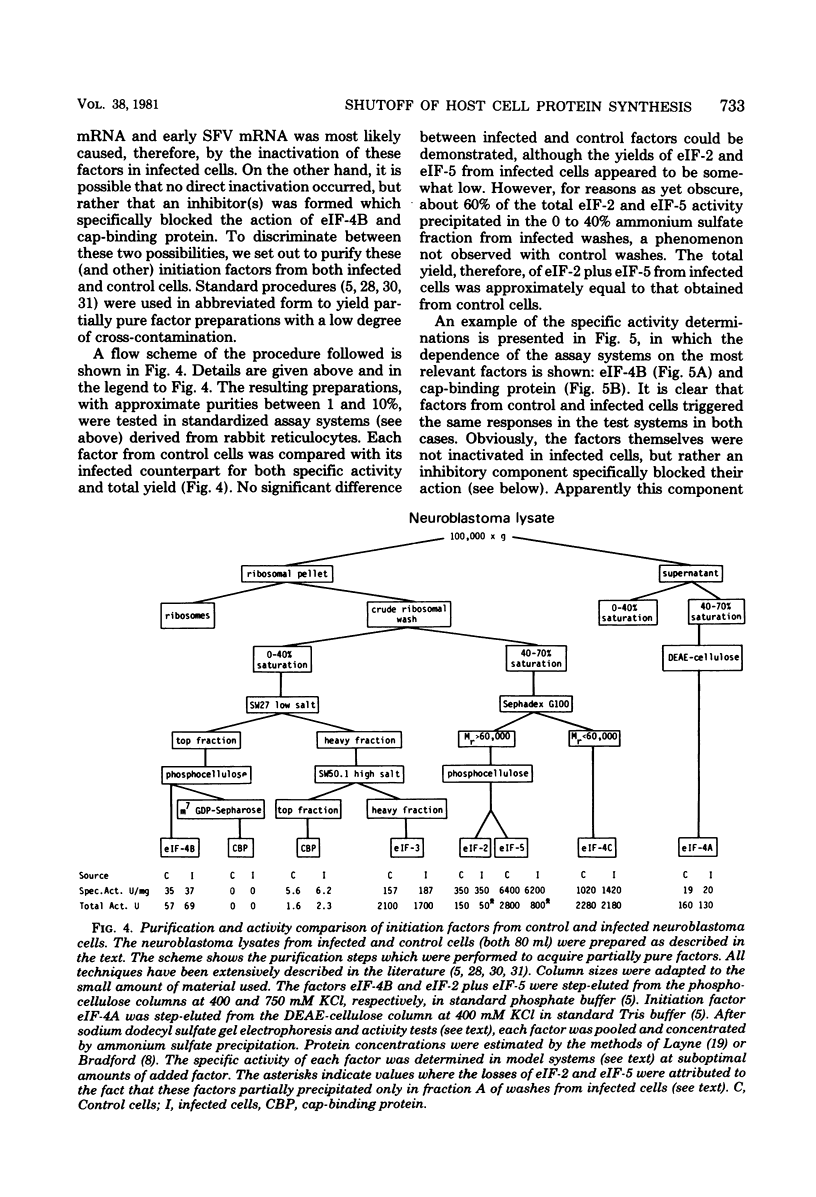
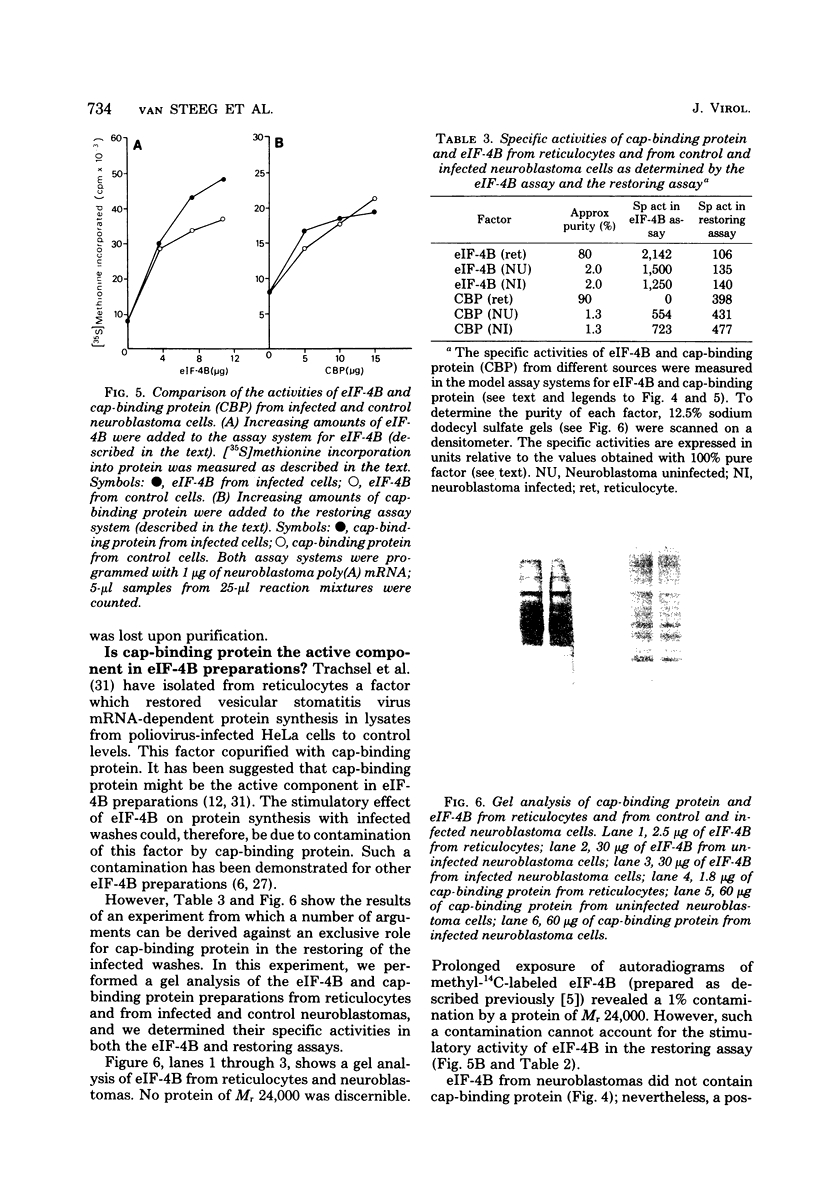
A crude ribosomal wash containing the initiation factors of protein synthesis was isolated from mouse neuroblastoma cells 8 h after infection with Semliki Forest virus (SFV). The activity of this wash was compared with that of a wash from control cells in a cell-free protein-synthesizing “pH5” system, with early SFV mRNA (42S), late SFV mRNA (26S), encephalomyocarditis virus (EMC) mRNA, or neuroblastoma polyadenylated mRNA templates. A pronounced loss of activity (±80%) of the crude ribosomal wash from infected cells was observed with host mRNA (neuroblastoma polyadenylated mRNA) and early SFV mRNA, messengers which contain a cap structure at the 5′ terminus. However, these washes were only slightly less active in systems programmed with (noncapped) EMC mRNA and late SFV mRNA. Although late SFV mRNA (26S) is capped, the synthesis of late (= structural) proteins in infected lysates was insensitive to inhibition by cap analogs. Purified initiation factors eIF-4B (Mr, 80,000) and cap-binding protein (Mr, 24,000) from reticulocytes (but none of the others) were able to restore the activity of infected factors to about 90% of control levels in systems programmed with early SFV mRNA and host mRNA. These observations indicate that infection-exposed crude initiation factors have a decreased level of eIF-4B and cap-binding protein activity. However, after partial purification of these and other initiation factors from infected and control cells, we found no significant difference in activity when model assay systems were used. Furthermore, both eIF-4B and cap-binding protein from infected cells were able to restore the activity of these infection-exposed factors to the same level obtained when these factors isolated from control cells or reticulocytes were added. A possible mechanism for the shutoff of host cell protein synthesis is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz H., Goumans H., Haubrich-Morree T., Voorma H. O., Benne R. Purification and characterization of a protein factor that reverses the inhibition of protein synthesis by the heme-regulated translational inhibitor in rabbit reticulocyte lysates. Eur J Biochem. 1979 Aug 1;98(2):513–520. doi: 10.1111/j.1432-1033.1979.tb13212.x. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., Bosch L., Cohn W. E., Lodish H., Merrick W. C., Weissbach H., Wittmann H. G., Wool I. G. International symposium on protein synthesis. Summary of Fogarty Center-NIH Workshop held in Bethesda, Maryland on 18-20 October, 1976. FEBS Lett. 1977 Apr 1;76(1):1–10. doi: 10.1016/0014-5793(77)80109-9. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Simili M., Shafritz D. A. Initiation activity of EMC virus RNA, binding to initiation factor eIF-4B and shut-off of host cell protein synthesis. Nature. 1978 Sep 21;275(5677):240–243. doi: 10.1038/275240a0. [DOI] [PubMed] [Google Scholar]
- Balanian R. Structural and functional alterations in cultured cells infected with cytocidal viruses. Prog Med Virol. 1975;19:40–83. [PubMed] [Google Scholar]
- Bergmann J. E., Trachsel H., Sonenberg N., Shatkin A. J., Lodish H. F. Characterization of rabbit reticulocyte factor(s) that stimulates the translation of mRNAs lacking 5'-terminal 7-methylguanosine. J Biol Chem. 1979 Mar 10;254(5):1440–1443. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chroboczek J., Puchkova L., Zagórski W. Regulation of brome mosaic virus gene expression by restriction of initiation of protein synthesis. J Virol. 1980 May;34(2):330–335. doi: 10.1128/jvi.34.2.330-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Initiation of synthesis of the structural proteins of Semliki Forest virus. J Mol Biol. 1975 Oct 5;97(4):401–411. doi: 10.1016/s0022-2836(75)80050-7. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., Burke D. C. Proteins synthesized in chick cells following infection with Semliki Forest virus. J Gen Virol. 1968 Sep;3(2):175–184. doi: 10.1099/0022-1317-3-2-175. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Control of protein synthesis in extracts from poliovirus-infected cells. I. mRNA discrimination by crude initiation factors. J Virol. 1978 May;26(2):510–521. doi: 10.1128/jvi.26.2.510-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Proteins synthesized by Semliki Forest virus and its 16 temperature-sensitive mutants. J Virol. 1975 Aug;16(2):388–396. doi: 10.1128/jvi.16.2.388-396.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Padilla M., Canaani D., Groner Y., Weinstein J. A., Bar-Joseph M., Merrick W., Shafritz D. A. Initiation factor eIF-4B (IF-M3)-dependent recognition and translation of capped versus uncapped eukaryotic mRNAs. J Biol Chem. 1978 Sep 10;253(17):5939–5945. [PubMed] [Google Scholar]
- Pettersson R. F., Söderlund H., Käriäinen L. The nucleotide sequences of the 5'-terminal T1 oligonucleotides of Semliki-Forest-virus 42-S and 26-S RNAs are different. Eur J Biochem. 1980 Apr;105(3):435–443. doi: 10.1111/j.1432-1033.1980.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Weinstein J. A., Safer B., Merrick W. C., Weber L. A., Hickey E. D., Baglioni C. Evidence for role of m7G5'-phosphate group in recognition of eukaryotic mRNA by initiation factor IF-M3. Nature. 1976 May 27;261(5558):291–294. doi: 10.1038/261291a0. [DOI] [PubMed] [Google Scholar]
- Skup D., Millward S. Highly efficient translation of messenger RNA in cell-free extracts prepared from L-cells. Nucleic Acids Res. 1977 Oct;4(10):3581–3587. doi: 10.1093/nar/4.10.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Millward S. Reovirus-induced modification of cap-dependent translation in infected L cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):152–156. doi: 10.1073/pnas.77.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Goumans H., Amesz H., Benne R., Voorma H. O. A comparison of the initiation factors of eukaryotic protein synthesis from ribosomes and from the postribosomal supernatant. Eur J Biochem. 1979 Aug 1;98(2):329–337. doi: 10.1111/j.1432-1033.1979.tb13192.x. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi K., Kädäridäinen L., Söderlund H. Quantitation of Semlike Forest virus RNAs in infected cells using 32-P equilibrium labelling. Nucleic Acids Res. 1975 Apr;2(4):555–565. doi: 10.1093/nar/2.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G. Comparative studies on polyribosomal, nonpolyribosome-associated and viral 42 S RNA from BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jun;65(2):601–605. doi: 10.1016/0042-6822(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Wengler G. Protein synthesis in BHK-21 cells infected with semliki forest virus. J Virol. 1975 Jan;17(1):10–19. doi: 10.1128/jvi.17.1.10-19.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H., Pranger M. H., van der Zeijst B. A., Benne R., Voorma H. O. In vitro translation of Semliki Forest virus 42 S RNA: initiation at two different sites. FEBS Lett. 1979 Dec 1;108(1):292–298. doi: 10.1016/0014-5793(79)81231-4. [DOI] [PubMed] [Google Scholar]