Abstract
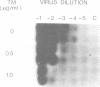
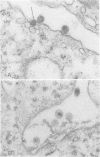
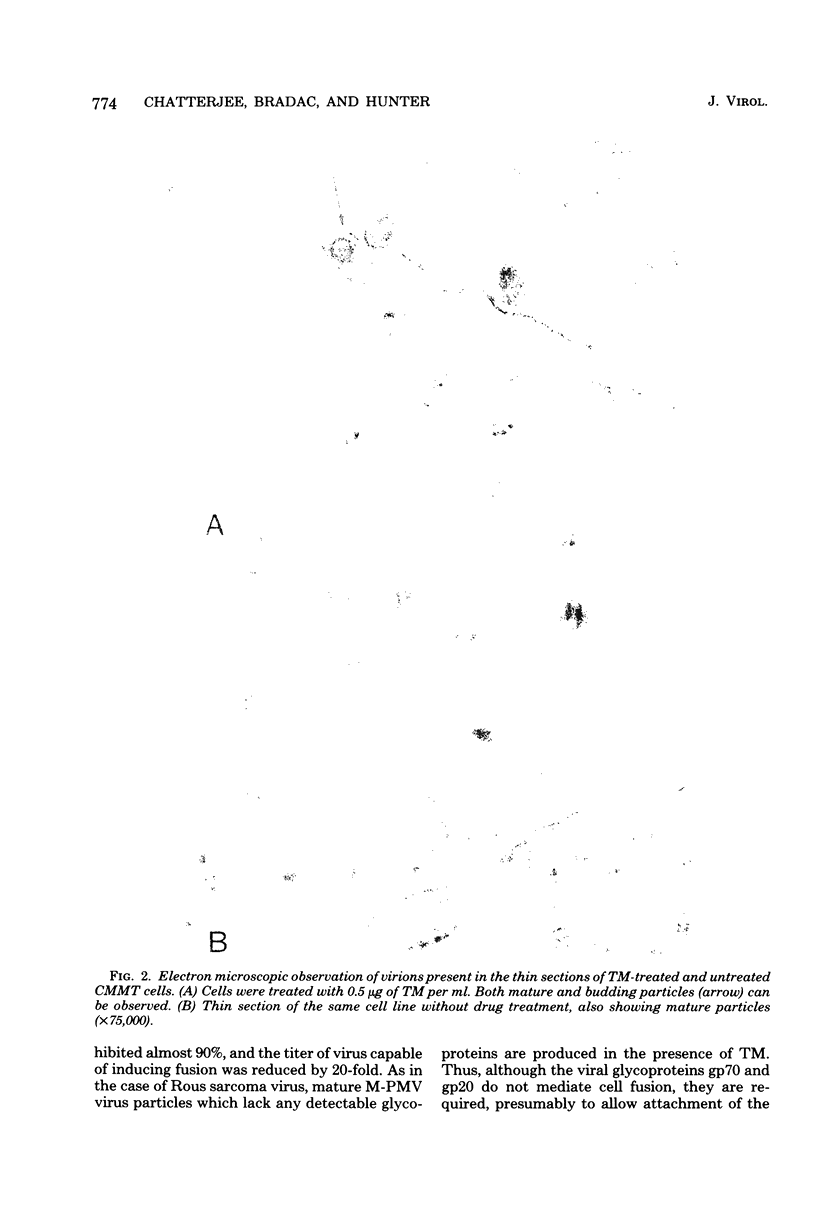
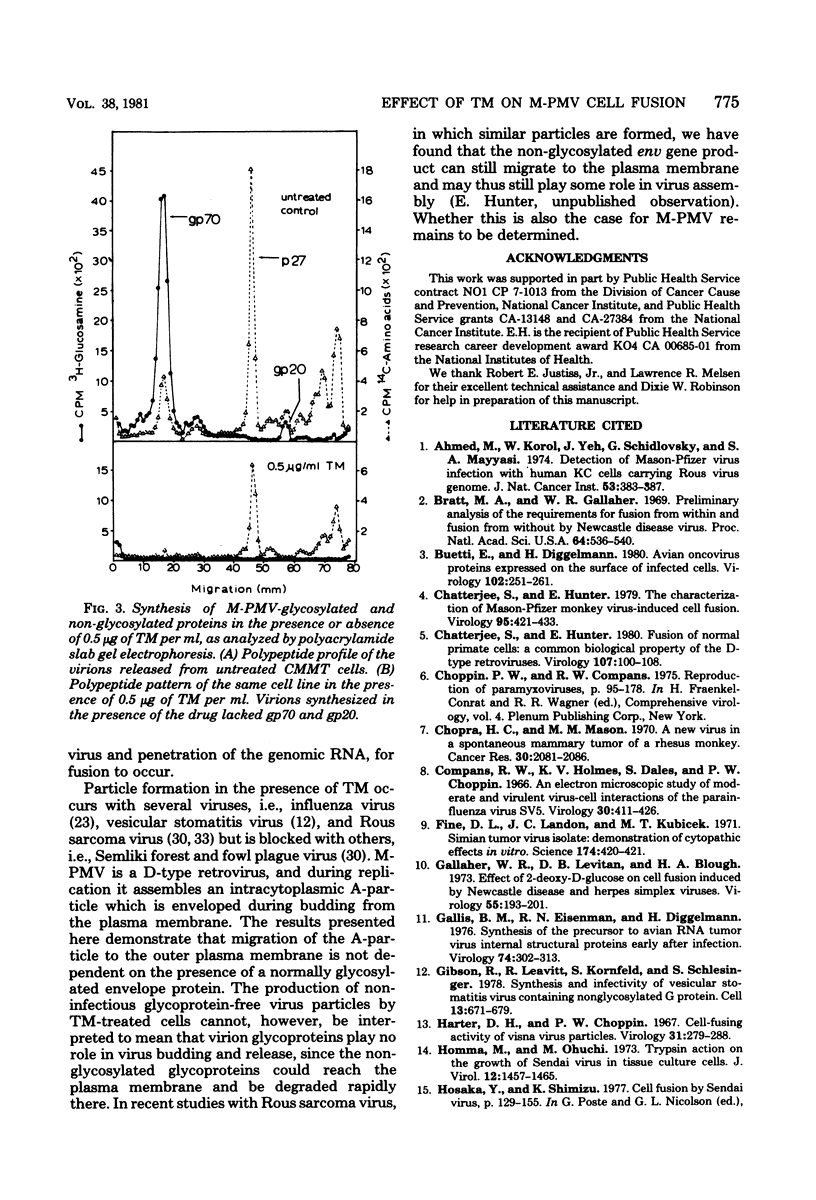
Mason-Pfizer monkey virus, a D-type retrovirus, has been shown to induce multinucleate cell (syncytium) formation or cell fusion in several normal primate cells. A series of experiments has been carried out to examine whether a glycosylated "fusion-inducing" product is responsible for this biological property of Mason-Pfizer monkey virus. Treatment of rhesus monkey fetal lung cells with different concentrations of tunicamycin, a potent inhibitor of glycosylation, during infection with Mason-Pfizer monkey virus had no effect on cell fusion even though up to 5 micrograms of the drug per ml was tested. Furthermore, no significant effect on the extent of syncytium formation in rhesus monkey fetal lung cells was observed when the time of addition or duration of treatment with this inhibitor was varied. Nevertheless, tunicamycin was very effective in blocking glycosylation in rhesus cells since virions produced in the presence of this drug completely lacked gp70 and gp20, the two structural glycoproteins of Mason-Pfizer monkey virus. These non-glycosylated virus particles produced in the presence of tunicamycin were noninfectious as determined by a protein A binding assay and were unable to induce syncytium formation when assayed on rhesus cells. These results indicate that glycosylation of the fusion-inducing product is not required for multinucleate cell formation induced by Mason Pfizer monkey virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Korol W., Yeh J., Schidlovsky G., Mayyasi S. A. Detection of Mason-Pfizer virus infection with human KC cells carrying Rous virus genome. J Natl Cancer Inst. 1974 Aug;53(2):383–387. doi: 10.1093/jnci/53.2.383. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Avian oncovirus proteins expressed on the surface of infected cells. Virology. 1980 Apr 30;102(2):251–261. doi: 10.1016/0042-6822(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. Fusion of normal primate cells: a common biological property of the D-type retroviruses. Virology. 1980 Nov;107(1):100–108. doi: 10.1016/0042-6822(80)90276-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. The characterization of Mason-Pfizer monkey virus-induced cell fusion. Virology. 1979 Jun;95(2):421–433. doi: 10.1016/0042-6822(79)90497-5. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Landon J. C., Kubicek M. T. Simian tumor virus isolate: demonstration of cytopathic effects in vitro. Science. 1971 Oct 22;174(4007):420–421. doi: 10.1126/science.174.4007.420. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Levitan D. B., Blough H. A. Effect of 2-deoxy-D-glucose on cell fusion induced by Newcastle disease and herpes simplex viruses. Virology. 1973 Sep;55(1):193–201. doi: 10.1016/s0042-6822(73)81021-9. [DOI] [PubMed] [Google Scholar]
- Gallis B. M., Eisenman R. N., Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976 Oct 15;74(2):302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. V., Dille B. J., Thimmig R. L., Johnson T. C., Rabinowitz S. G., Dal Canto M. C. Neuroblastoma cell fusion by a temperature-sensitive mutant of vesicular stomatitis virus. J Virol. 1979 Jun;30(3):883–890. doi: 10.1128/jvi.30.3.883-890.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Hayman M. J., Rongey R. W., Vogt P. K. An avian sarcoma virus mutant that is temperature sensitive for virion assembly. Virology. 1976 Jan;69(1):35–49. doi: 10.1016/0042-6822(76)90192-6. [DOI] [PubMed] [Google Scholar]
- KOHN A. POLYKARYOCYTOSIS INDUCED BY NEWCASTLE DISEASE VIRUS IN MONOLAYERS OF ANIMAL CELLS. Virology. 1965 Jun;26:228–245. doi: 10.1016/0042-6822(65)90050-4. [DOI] [PubMed] [Google Scholar]
- Keller J. M. The expression of the syn- gene of herpes simplex virus type 1. II. Requirements for macromolecular synthesis. Virology. 1976 Jul 15;72(2):402–409. doi: 10.1016/0042-6822(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J., Nowinski R. C. Identification of the Gross cell surface antigen associated with murine leukemia virus-infected cells. J Virol. 1977 Aug;23(2):315–322. doi: 10.1128/jvi.23.2.315-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J. Biological properties of syncytium-forming ("foamy") viruses. Virology. 1972 Mar;47(3):673–683. doi: 10.1016/0042-6822(72)90557-0. [DOI] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Poste G. Virus-induced polykaryocytosis and the mechanism of cell fusion. Adv Virus Res. 1970;16:303–356. doi: 10.1016/s0065-3527(08)60026-3. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Long C. W. Fusion of a Rous sarcoma virus transformed human cell line, KC, by RD-114 virus. J Gen Virol. 1973 Dec;21(3):523–532. doi: 10.1099/0022-1317-21-3-523. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtz R., Dolev S., Aboud M., Salzberg S. Viral genome RNA serves as messenger early in the infectious cycle of murine leukemia virus. J Virol. 1979 Sep;31(3):668–676. doi: 10.1128/jvi.31.3.668-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Stockert E., Fleissner E. Characterization of molecular species carrying gross cell surface antigen. J Virol. 1977 Aug;23(2):302–314. doi: 10.1128/jvi.23.2.302-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Shimizu K. I., Tamura G. Effect of tunicamycin on microorganisms: morphological changes and degradation of RNA and DNA induced by tunicamycin. J Antibiot (Tokyo) 1972 Feb;25(2):75–85. doi: 10.7164/antibiotics.25.75. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Tunicamycin, a new antibiotic. 3. Reversal of the antiviral activity of tunicamycin by aminosugars and their derivatives. J Antibiot (Tokyo) 1971 Apr;24(4):232–238. [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Tunicamycin, a new antibiotic. II. Some biological properties of the antiviral activity of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):224–231. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Induction of syncytia by Moloney murine leukemia virus in myoblasts defective in differentiation. J Virol. 1977 Jan;21(1):319–327. doi: 10.1128/jvi.21.1.319-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Mechanism of murine leukemia virus-induced fusion in rat myoblasts defective in differentiation. J Virol. 1977 Sep;23(3):768–775. doi: 10.1128/jvi.23.3.768-775.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]