Abstract
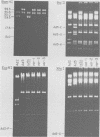
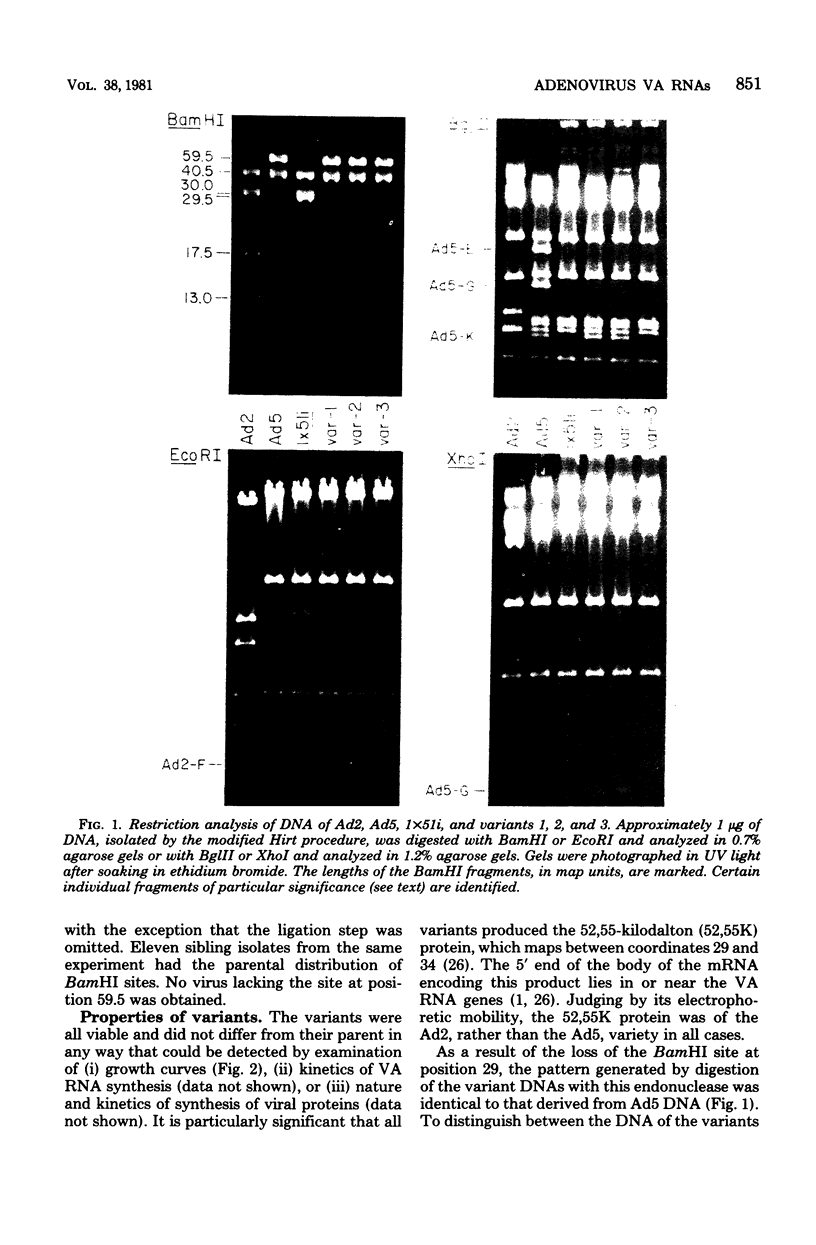
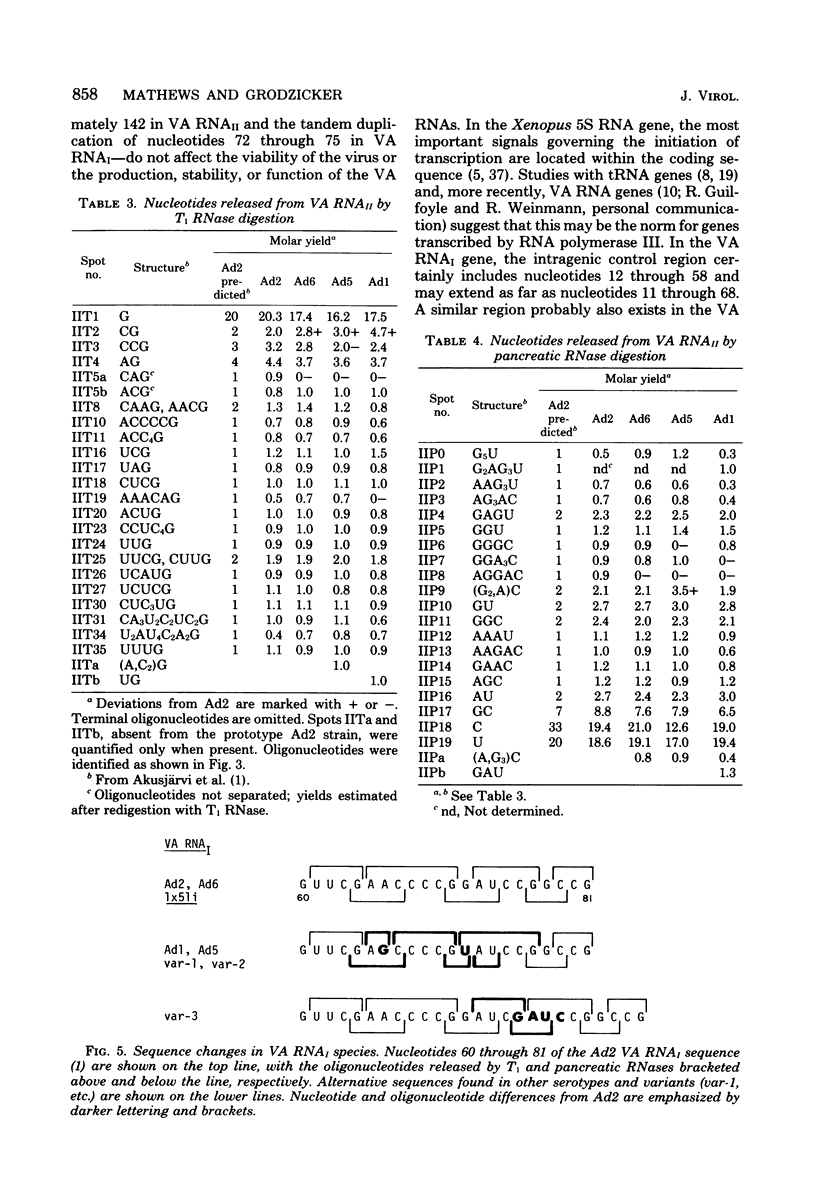
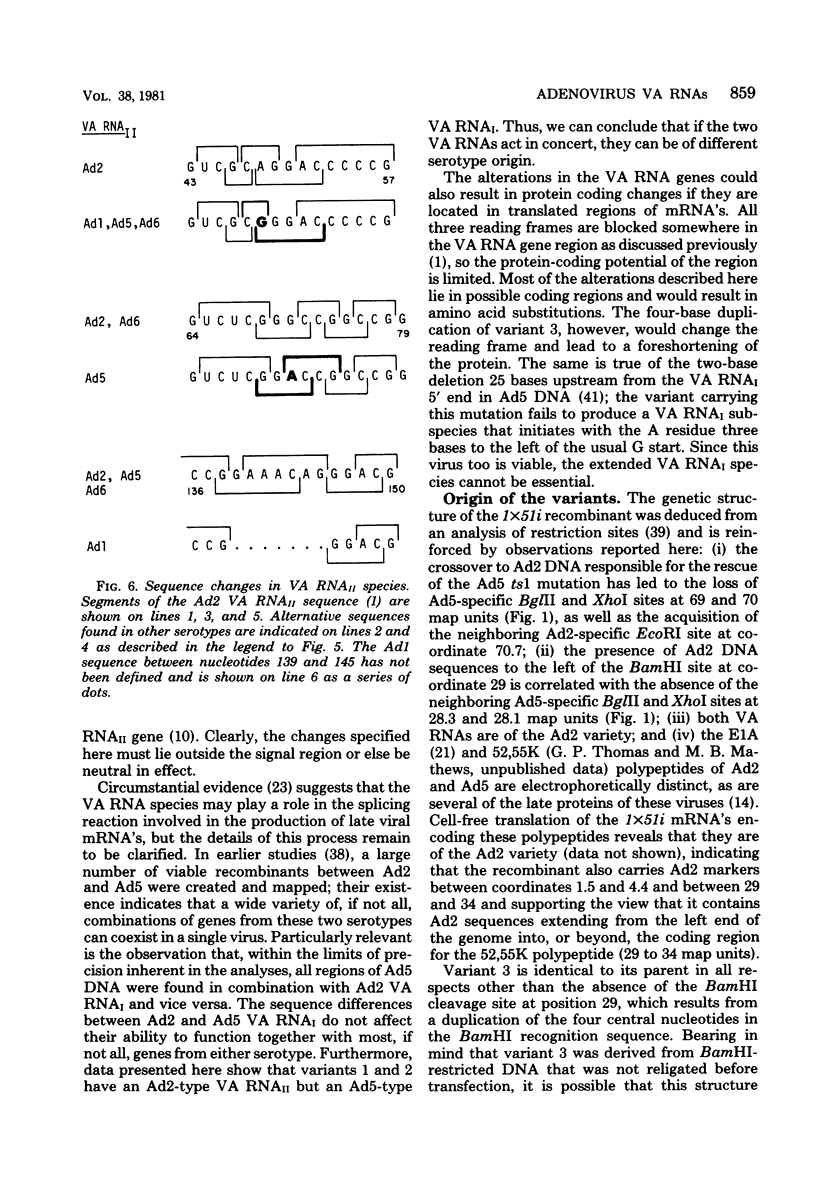
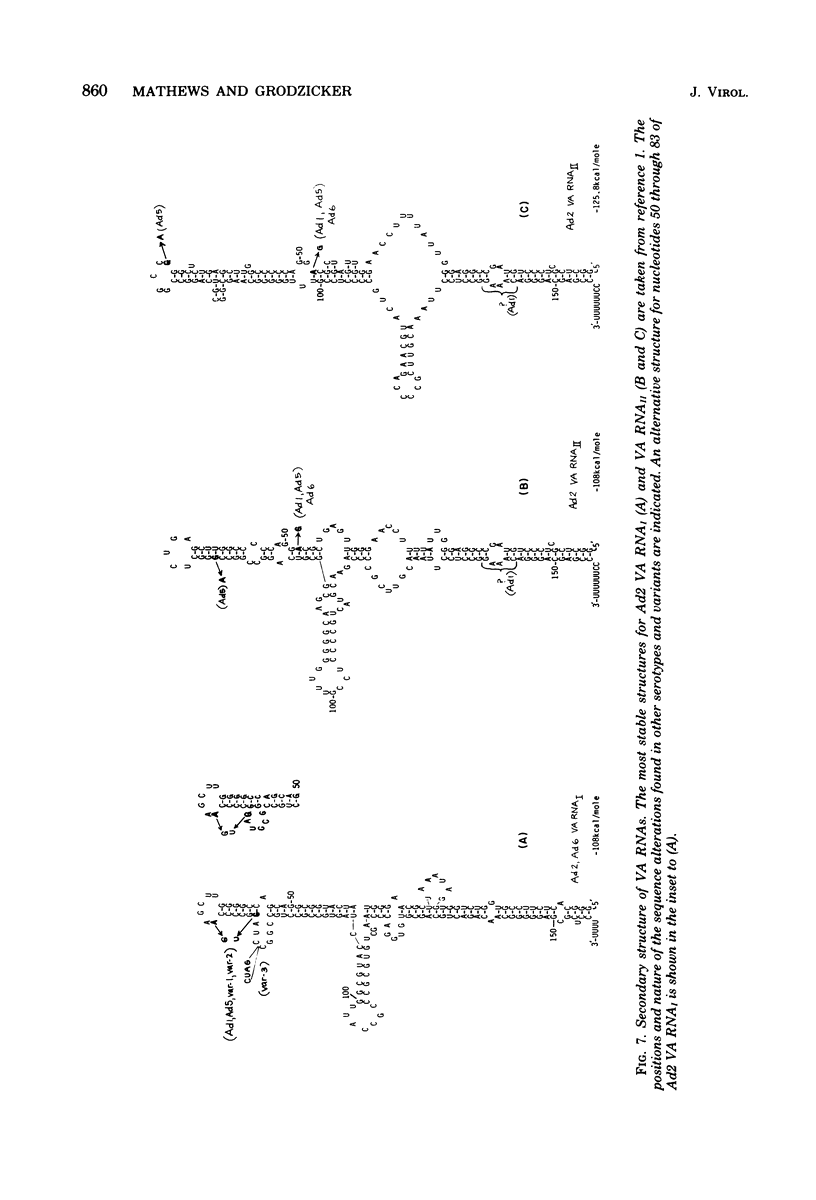
We compared the sequences of the virus-associated (VA) RNAs of group C adenoviruses, serotypes 1, 2, 5, and 6, and of three variants of adenovirus type 2 (Ad2) selected for loss of the BamHI restriction site in the VA RNAI gene. In the naturally occurring strains. VA RNAI exists in two forms which differ by two nucleotides: one form is found in Ad2 and Ad6, and the other is found in Ad1 and Ad5. There are three sites of variation in Va RNAII, the Ad1, Ad2, and Ad5 forms each differing from Ad6 VA RNAII at one of the positions. One of the selected variants has a four-base duplication within the BamHI cleavage site, whereas the two others have acquired a VA RNAI sequence indistinguishable from that of Ad5. The findings are interpreted in terms of the secondary structures of the VA RNAs and the interrelationships among the viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. I. The sequences at the 3' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9032–9042. [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. II. Heterogeneity at the 5' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9043–9046. [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco D., Schmidt O., Söll D. Two control regions for eukaryotic tRNA gene transcription. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3365–3368. doi: 10.1073/pnas.77.6.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Föhring B., Geis A., Koomey M., Raska K., Jr Adenovirus type 12 VA RNA. I. Synthesis in productive infection and gene mapping. Virology. 1979 Jun;95(2):295–302. doi: 10.1016/0042-6822(79)90485-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sambrook J., Mathews M. B. The physical locations of structural genes in adenovirus DNA. Virology. 1977 Jul 1;80(1):111–126. doi: 10.1016/0042-6822(77)90384-1. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sharp P. A., Sambrook J. Conditional lethal mutants of adenovirus 2-simian virus 40 hybrids. I. Host range mutants of Ad2+ND1. J Virol. 1974 Jun;13(6):1237–1244. doi: 10.1128/jvi.13.6.1237-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B., Roeder R. G. Structural relationships of low molecular weight viral RNAs synthesized by RNA polymerase III in nuclei from adenovirus 2-infected cells. J Biol Chem. 1978 Jun 25;253(12):4120–4127. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Hofstetter H., Di Capua E., Grosschedl R., Birnstiel M. L. A tRNA gene of Xenopus laevis contains at least two sites promoting transcription. Nucleic Acids Res. 1979 Dec 11;7(7):1749–1763. doi: 10.1093/nar/7.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping the genes of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):53–60. doi: 10.1101/sqb.1974.039.01.010. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Esche H., Smart J. E., Stillman B. W., Harter M. L., Mathews M. B. Organization and expression of the left third of the genome of adenovirus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):493–508. doi: 10.1101/sqb.1980.044.01.052. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Binding of adenovirus VA RNA to mRNA: a possible role in splicing? Nature. 1980 Jun 19;285(5766):575–577. doi: 10.1038/285575a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Genes for VA-RNA in adenovirus 2. Cell. 1975 Oct;6(2):223–229. doi: 10.1016/0092-8674(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Pettersson U. The low molecular weight of RNAs of adenovirus 2-infected cells. J Mol Biol. 1978 Feb 25;119(2):293–328. doi: 10.1016/0022-2836(78)90439-4. [DOI] [PubMed] [Google Scholar]
- McGuire P. M., Piatak M., Hodge L. D. Nuclear and cytoplasmic adenovirus RNA. Differences between 5'-terminal of messenger and non-messenger transcripts. J Mol Biol. 1976 Mar 5;101(3):379–396. doi: 10.1016/0022-2836(76)90154-6. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Murray V., Holliday R. Mechanism for RNA splicing of gene transcripts. FEBS Lett. 1979 Oct 1;106(1):5–7. doi: 10.1016/0014-5793(79)80682-1. [DOI] [PubMed] [Google Scholar]
- Ohe K. Virus-coded origin of a low molecular weight RNA from KB cells infected with adenovirus 2. Virology. 1972 Mar;47(3):726–733. doi: 10.1016/0042-6822(72)90562-4. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M., Cooke N. R. Studies on the origin of a low molecular weight ribonucleic acid from human cells infected with adenoviruses. J Biol Chem. 1969 Oct 10;244(19):5320–5332. [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. The nucleotide sequence of a low molecular weight ribonucleic acid from cells infected with adenovirus 2. J Biol Chem. 1971 Nov 25;246(22):6991–7009. [PubMed] [Google Scholar]
- Pan J., Celma M. L., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. III. The sequence of the promoter for VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9047–9054. [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Location of sequences on the adenovirus genome coding for the 5.5S RNA. Cell. 1975 Sep;6(1):1–4. doi: 10.1016/0092-8674(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Forget B. G., Weissman S. M. RNA of low molecular weight in KB cells infected with adenovirus type 2. J Mol Biol. 1966 Jun;17(2):428–439. doi: 10.1016/s0022-2836(66)80153-5. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sleigh M., Engler J. A., Broker T. R. The evolution of the adenoviral genome. Ann N Y Acad Sci. 1980;354:426–452. doi: 10.1111/j.1749-6632.1980.tb27983.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Jones N., Shenk T. A mutation which alters initiation of transcription by RNA polymerase III on the Ad5 chromosome. Cell. 1979 Dec;18(4):947–954. doi: 10.1016/0092-8674(79)90207-1. [DOI] [PubMed] [Google Scholar]
- Varricchio F., Raska K., Jr Synthesis of VA RNA in productive and abortive infections with adenovirus type 12. Virology. 1976 Oct 15;74(2):450–458. doi: 10.1016/0042-6822(76)90351-2. [DOI] [PubMed] [Google Scholar]
- Vennström B., Pettersson U., Philipson L. Two initiation sites for adenovirus 5.5S RNA. Nucleic Acids Res. 1978 Jan;5(1):195–204. doi: 10.1093/nar/5.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Brendler T. G., Raskas H. J., Roeder R. G. Low molecular weight viral RNAs transcribed by RNA polymerase III during adenovirus 2 infection. Cell. 1976 Apr;7(4):557–566. doi: 10.1016/0092-8674(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Gingeras T. R., Bullock P., Wong G., Gelinas R. E. Determination and analysis of adenovirus-2 DNA sequences which may include signals for late messenger RNA processing. J Mol Biol. 1979 Dec 5;135(2):413–433. doi: 10.1016/0022-2836(79)90444-3. [DOI] [PubMed] [Google Scholar]