Abstract
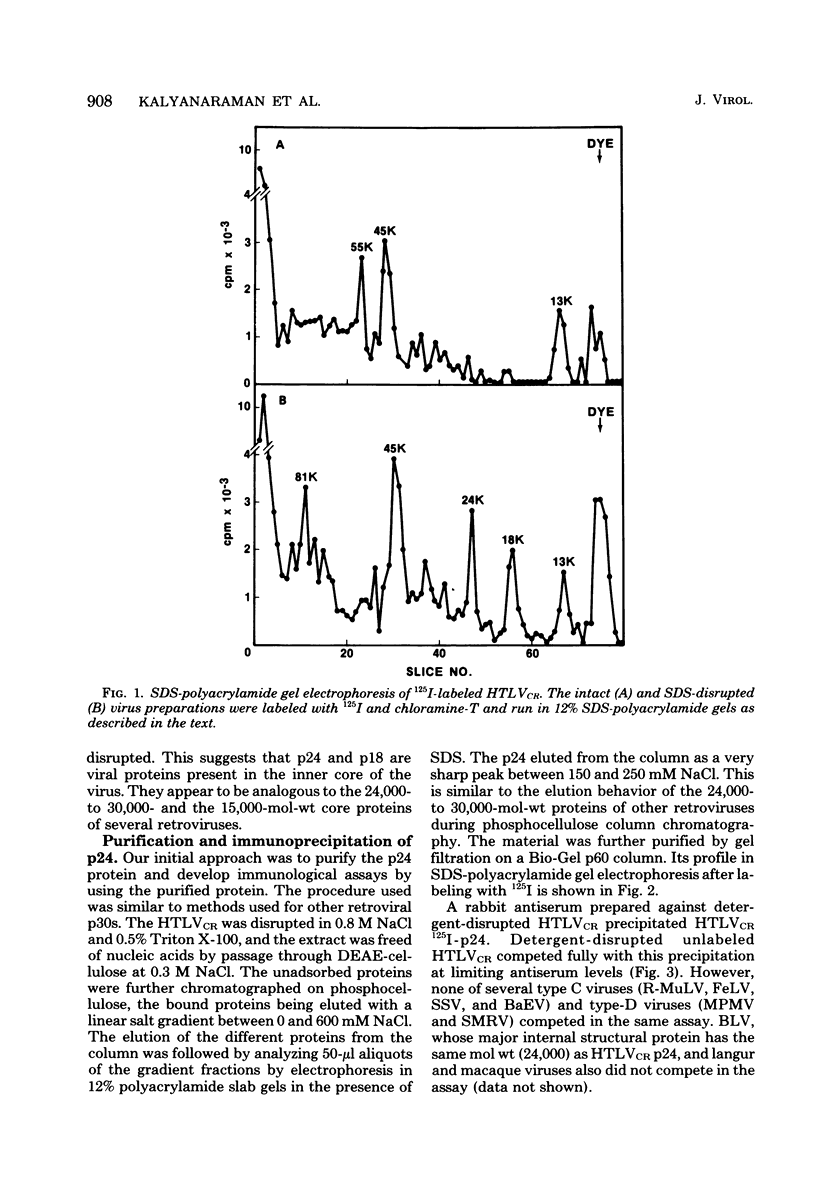
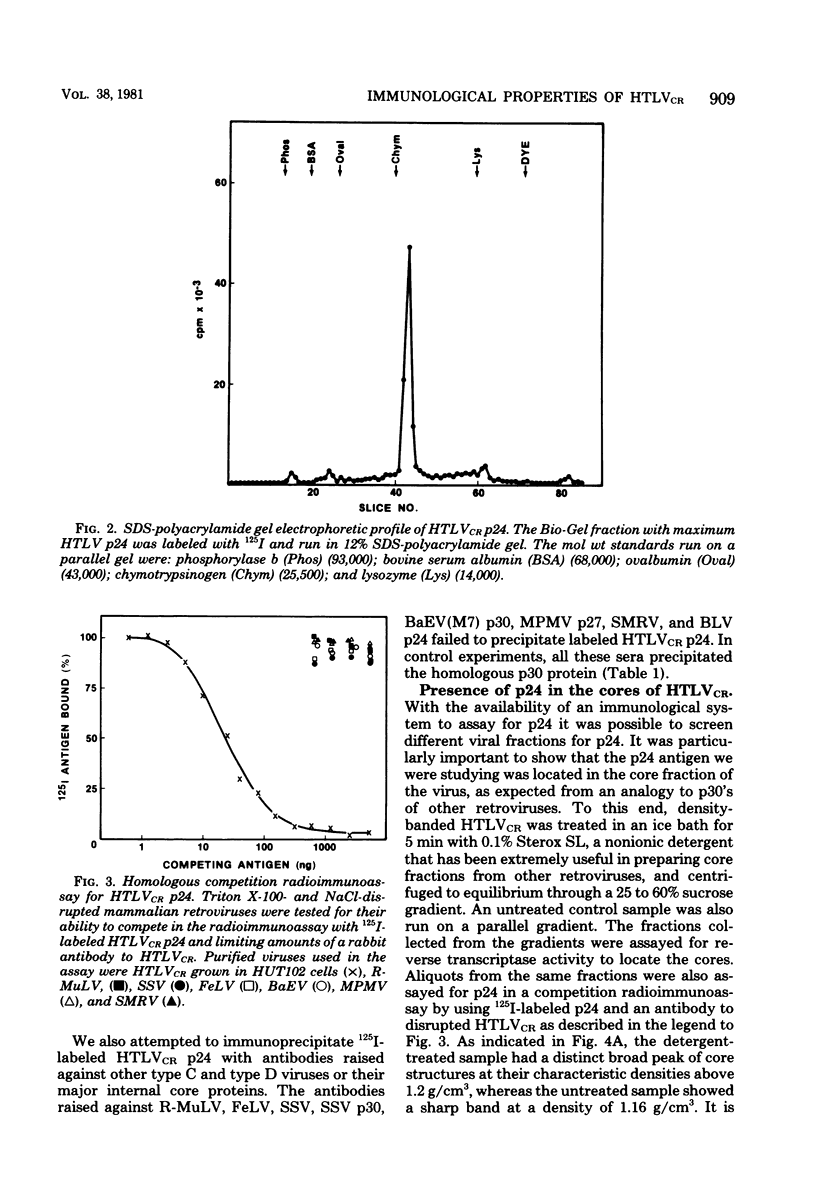
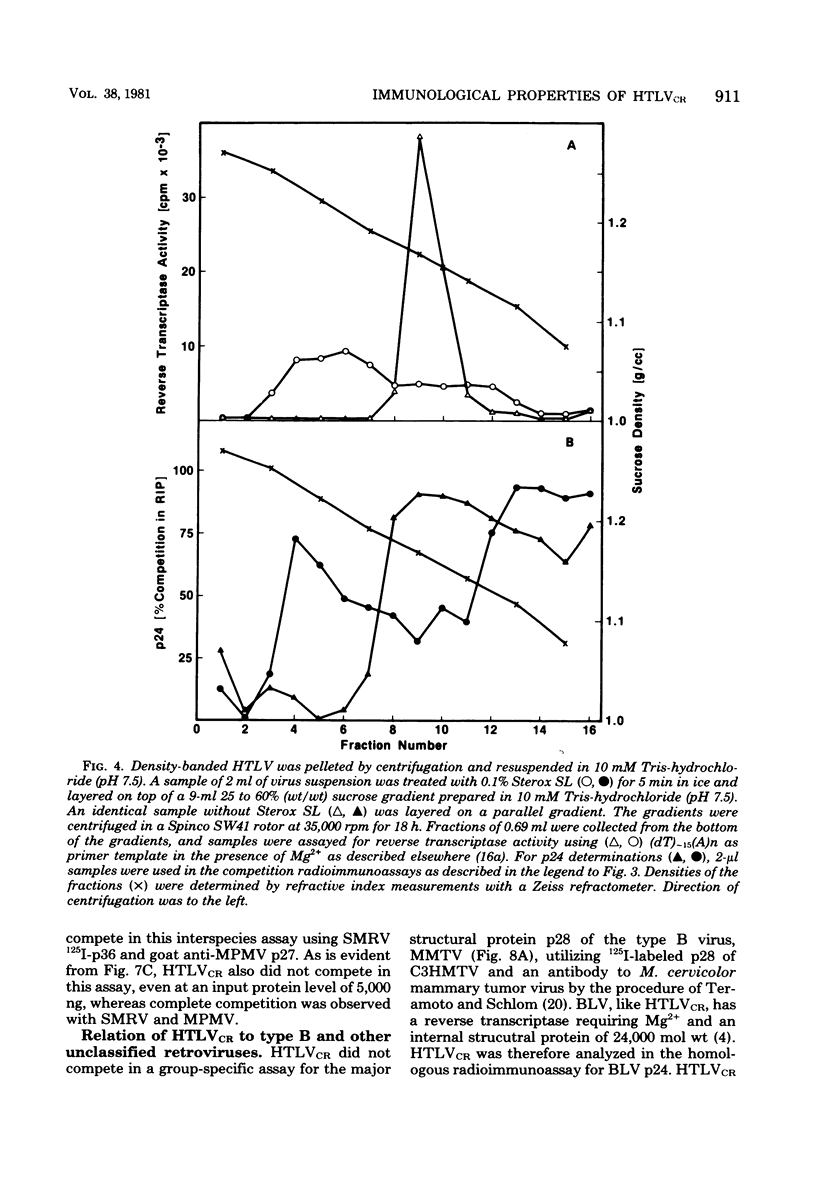
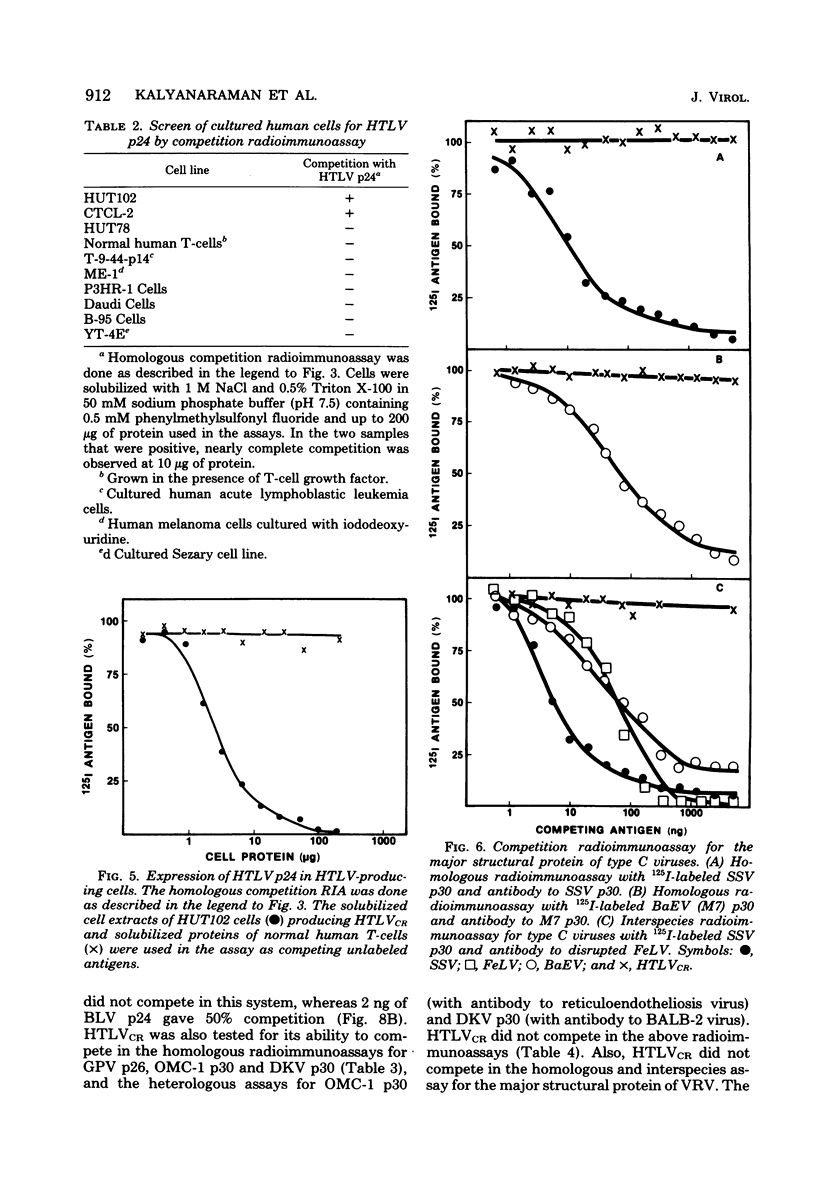
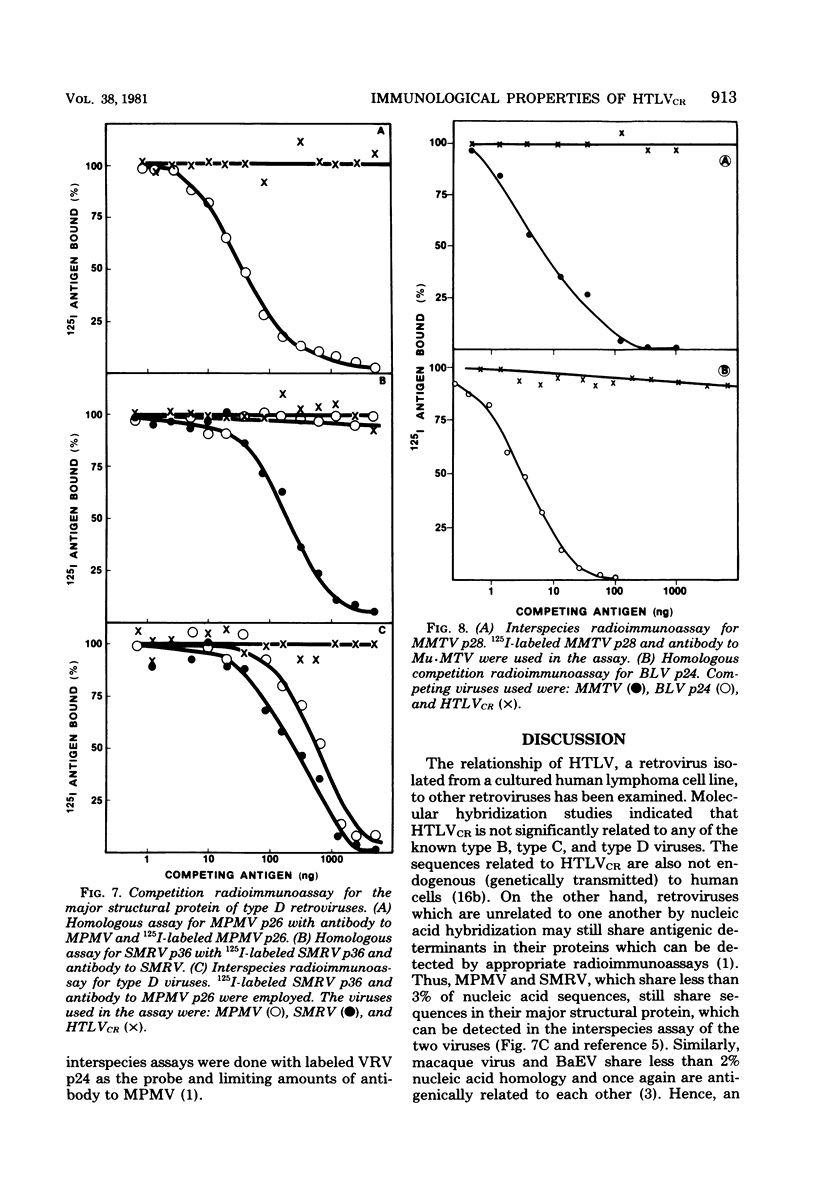
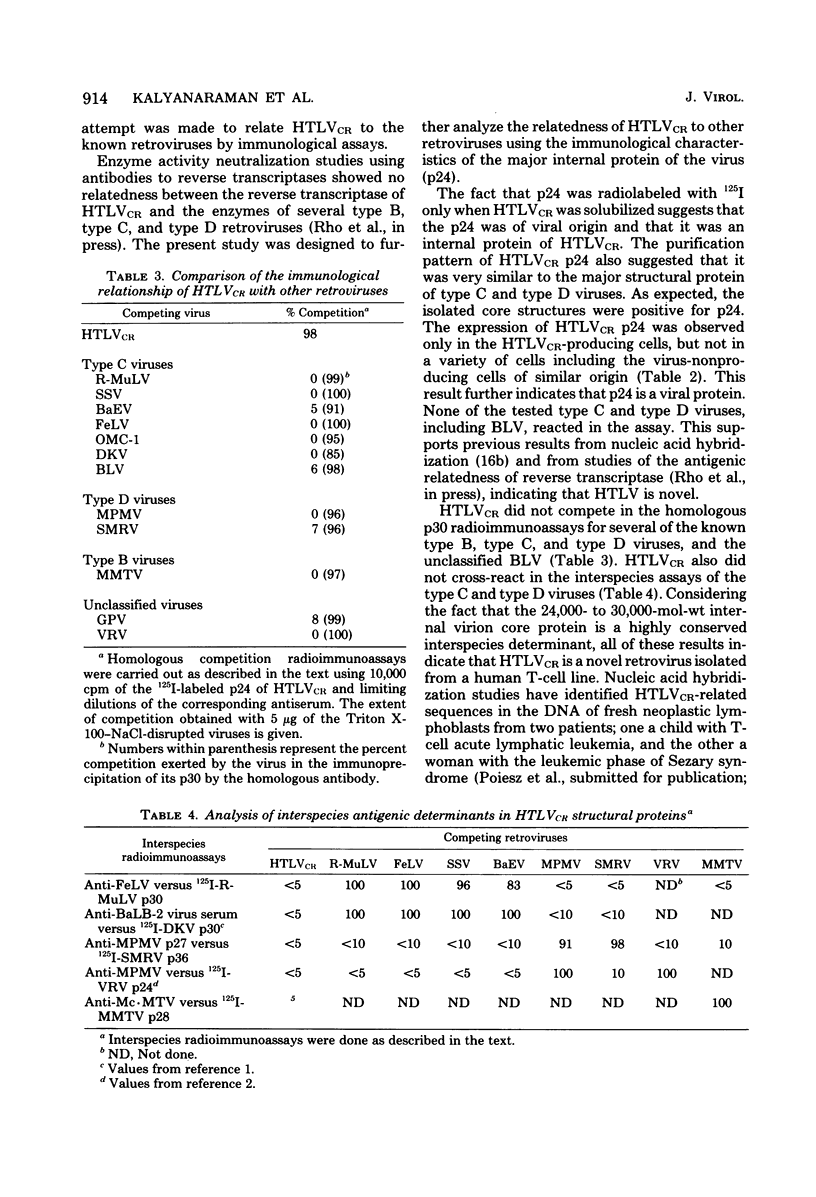
HTLV strain CR (HTLVCR) is a retrovirus which was isolated from a human T-cell lymphoma cell line. A protein of molecular weight 24,000 p24, was purified from this virus. Several results indicate that this p24 is an internal core protein of HTLVCR. (i) The p24 copurified with viral cores. (ii) It was labeled with 125I after disruption of the virus, but not when undisrupted virus was iodinated. (iii) The amount of p24 was directly proportional to the amount of HTLVCR. (iv) In chromatographic properties, the HTLVCR p24 behaved similarly to the major structural protein (24,000- to 30,000-molecular-weight protein) of other retroviruses. A rabbit antiserum raised against disrupted HTLVCR precipitated the labeled p24, and the precipitation was competed for by unlabeled HTLVCR and by cytoplasmic proteins from cells producing HTLVCR, but not by proteins from normal human cells, including normal growing human T-cells, and several cultured human cutaneous T-cell lymphoma lines. Proteins from several mammalian type B, type C, and type D viruses also failed to compete in this precipitation. Moreover, HTLVCR did not react in homologous and interspecies assays for p30 antigens of several mammalian type C and type D viruses. These observations agree with immunological comparisons between reverse transcriptase of HTLVCR and other retroviruses and nucleic acid sequence homology studies which indicate that the various HTLVCR isolates represent new retroviruses found in some human T-cell neoplasias.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Barbacid M., Tronick S. R., Clark H. F., Aaronson S. A. Evolutionary relatedness of viper and primate endogenous retroviruses. Science. 1979 Apr 20;204(4390):318–321. doi: 10.1126/science.219480. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Daniel M. D., Aaronson S. A. Immunological relationships of OMC-1, an endogenous virus of owl monkeys, with mammalian and avian type C viruses. J Virol. 1980 Jan;33(1):561–566. doi: 10.1128/jvi.33.1.561-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. L., Sherr C. J., Sen A., Todaro G. J. Molecular diversity among five different endogenous primate retroviruses. J Virol. 1978 Oct;28(1):300–313. doi: 10.1128/jvi.28.1.300-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Teramoto Y. A., Schlom J. Interspecies radioimmunoassay for the major structural proteins of primate type-D retroviruses. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5739–5743. doi: 10.1073/pnas.74.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Type C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science. 1975 Jan 31;187(4174):350–353. doi: 10.1126/science.46123. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Reitz M. S., Jr Molecular probes for tumor viruses in human cancer. Int Rev Exp Pathol. 1976;16:1–58. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Kaplan H. S., Goodenow R. S., Epstein A. L., Gartner S., Declève A., Rosenthal P. N. Isolation of a type C RNA virus from an established human histiocytic lymphoma cell line. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2564–2568. doi: 10.1073/pnas.74.6.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Panem S., Prochownik E. V., Reale F. R., Kirsten W. H. Isolation of type C virions from a normal human fibroblast strain. Science. 1975 Jul 25;189(4199):297–299. doi: 10.1126/science.49927. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Smith R. G., Nooter K., Bentvelzen P., Robert-Guroff M., Harewood K., Reitz M. S., Lee S. A., Gallo R. C. Characterization of a type-C virus produced by co-cultures of human leukemic bone-marrow and fetal canine thymus cells. Int J Cancer. 1979 Aug;24(2):210–217. doi: 10.1002/ijc.2910240213. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Teramoto Y. A., Schlom J. Radioimmunoassays that demonstrate type-specific and group-specific antigenic reactivities for the major internal structural protein of murine mammary tumor viruses. Cancer Res. 1978 Jul;38(7):1990–1995. doi: 10.1203/00006450-199501000-00002. [DOI] [PubMed] [Google Scholar]


