Abstract
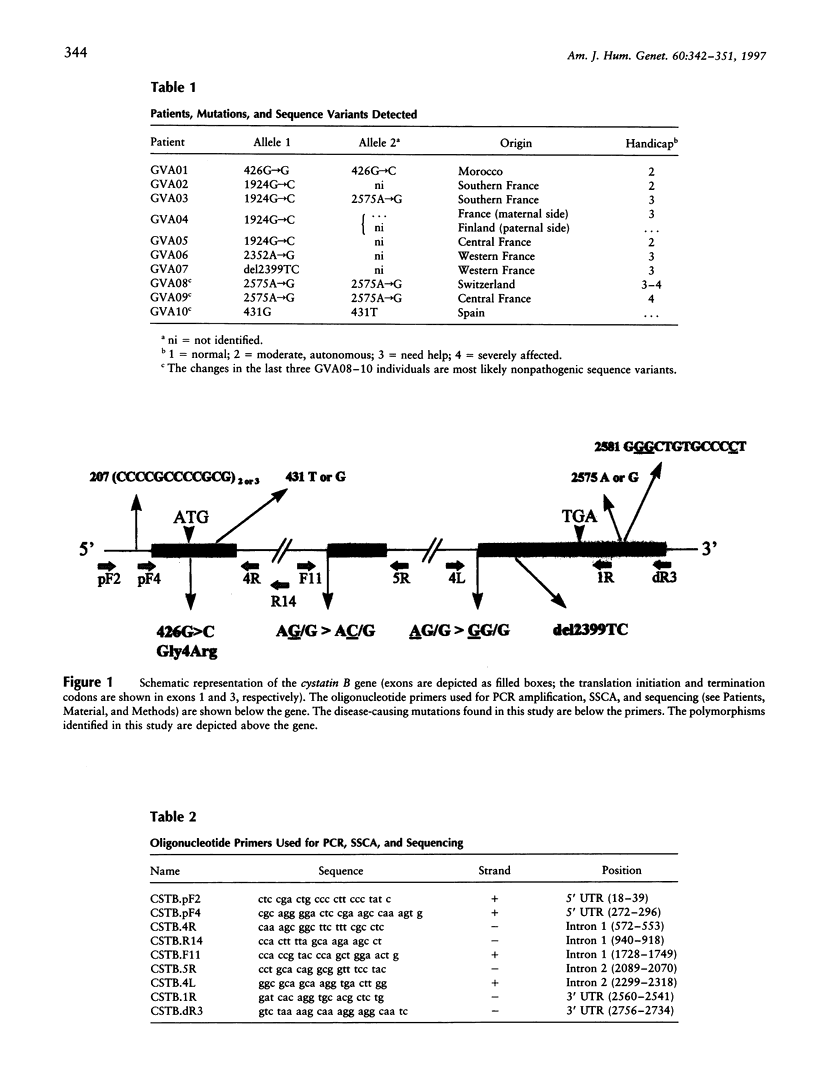
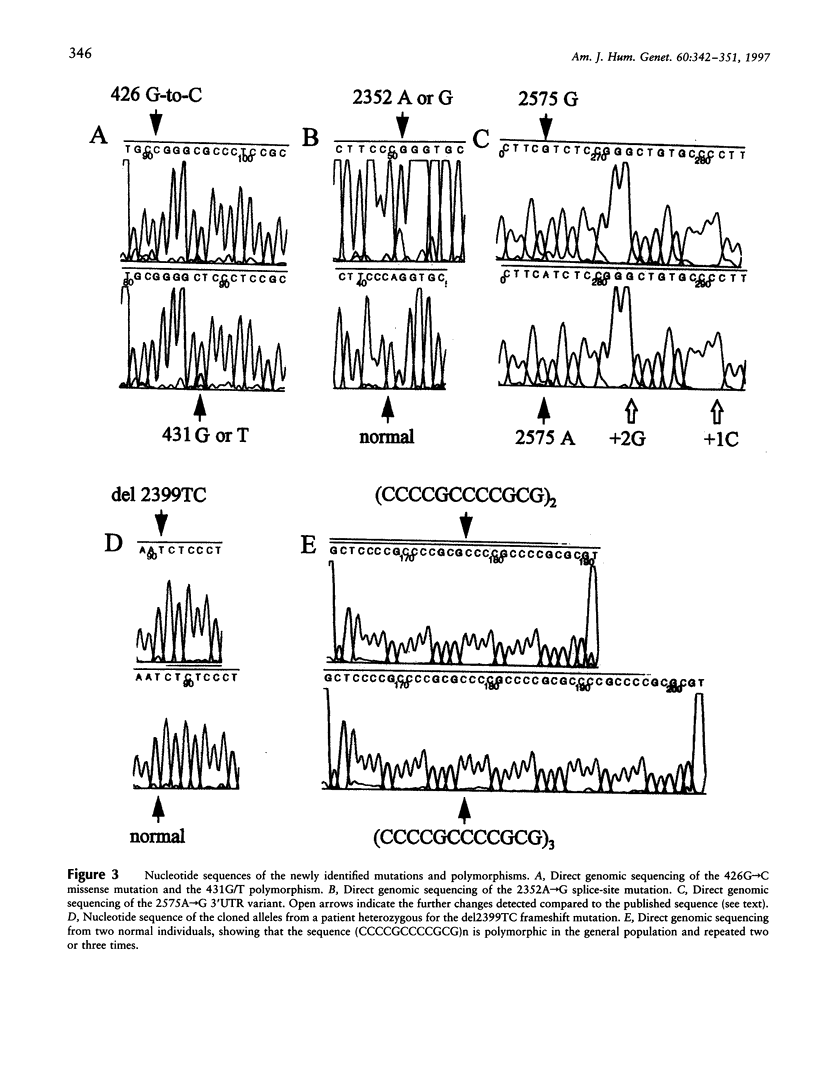
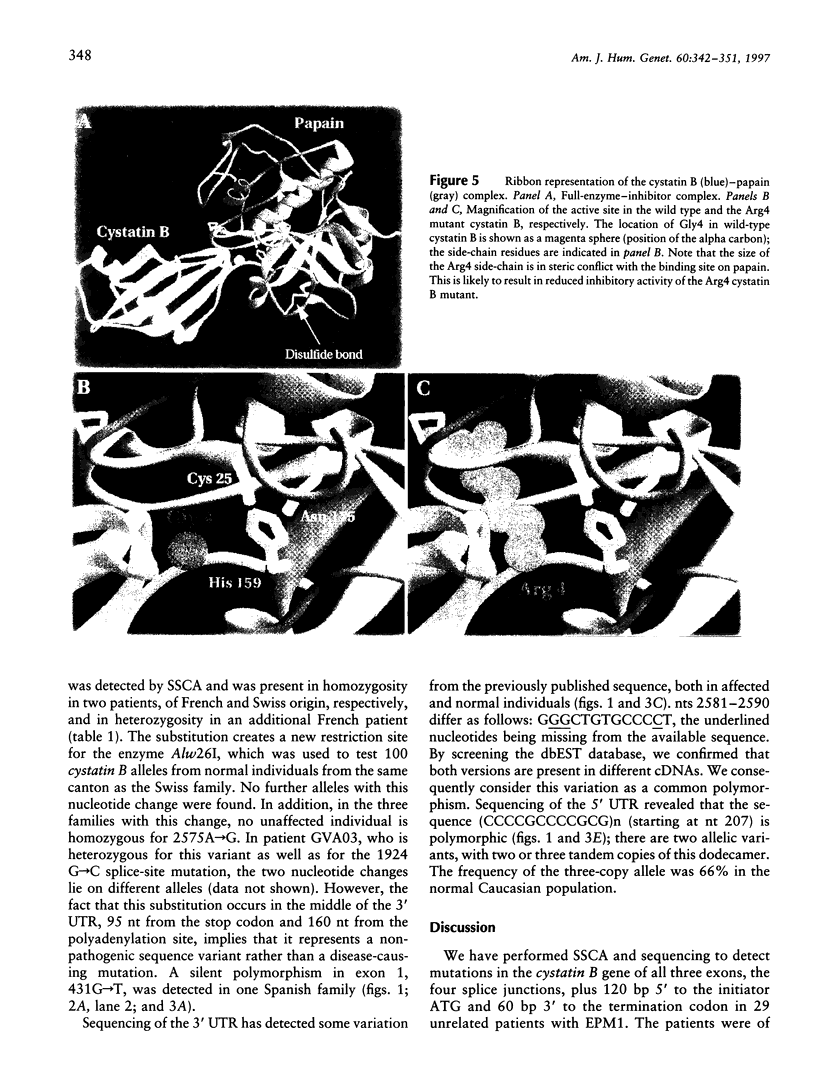
Progressive myoclonus epilepsy (EPM1) is an autosomal recessive disorder, characterized by severe, stimulus-sensitive myoclonus and tonic-clonic seizures. The EPM1 locus was mapped to within 0.3 cM from PFKL in chromosome 21q22.3. The gene for the proteinase inhibitor cystatin B was recently localized in the EPM1 critical region, and mutations were identified in two EPM1 families. We have identified six nucleotide changes in the cystatin B gene of non-Finnish EPM1 families from northern Africa and Europe. The 426G-->C change in exon 1 results in a Gly4Arg substitution and is the first missense mutation described that is associated with EPM1. Molecular modeling predicts that this substitution severely affects the contact of cystatin B with papain. Mutations in the invariant AG dinucleotides of the acceptor sites of introns 1 and 2 probably result in abnormal splicing. A deletion of two nucleotides in exon 3 produces a frameshift and truncates the protein. Therefore, these four mutations are all predicted to impair the production of functional protein. These mutations were found in 7 of the 29 unrelated EPM1 patients analyzed, in homozygosity in 1, and in heterozygosity in the others. The remaining two sequence changes, 431G-->T and 2575A-->G, probably represent polymorphic variants. In addition, a tandem repeat in the 5' UTR (CCCCGCCCCGCG) is present two or three times in normal alleles. It is peculiar that in the majority of patients no mutations exist within the exons and splice sites of the cystatin B gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Chumakov I., Rigault P., Guillou S., Ougen P., Billaut A., Guasconi G., Gervy P., LeGall I., Soularue P., Grinas L. Continuum of overlapping clones spanning the entire human chromosome 21q. Nature. 1992 Oct 1;359(6394):380–387. doi: 10.1038/359380a0. [DOI] [PubMed] [Google Scholar]
- Cochius J. I., Figlewicz D. A., Kälviäinen R., Nousiainen U., Farrell K., Patry G., Söderfeldt B., Frydman M., Lerman P., Andermann F. Unverricht-Lundborg disease: absence of nonallelic genetic heterogeneity. Ann Neurol. 1993 Nov;34(5):739–741. doi: 10.1002/ana.410340519. [DOI] [PubMed] [Google Scholar]
- Henskens Y. M., Veerman E. C., Nieuw Amerongen A. V. Cystatins in health and disease. Biol Chem Hoppe Seyler. 1996 Feb;377(2):71–86. doi: 10.1515/bchm3.1996.377.2.71. [DOI] [PubMed] [Google Scholar]
- Lafrenière R. G., de Jong P. J., Rouleau G. A. A 405-kb cosmid contig and HindIII restriction map of the progressive myoclonus epilepsy type 1 (EPM1) candidate region in 21q22.3. Genomics. 1995 Sep 1;29(1):288–290. doi: 10.1006/geno.1995.1248. [DOI] [PubMed] [Google Scholar]
- Lalioti M. D., Chen H., Rossier C., Shafaatian R., Reid J. D., Antonarakis S. E. Cloning the cDNA of human PWP2, which encodes a protein with WD repeats and maps to 21q22.3. Genomics. 1996 Jul 15;35(2):321–327. doi: 10.1006/geno.1996.0363. [DOI] [PubMed] [Google Scholar]
- Lehesjoki A. E., Eldridge R., Eldridge J., Wilder B. J., de la Chapelle A. Progressive myoclonus epilepsy of Unverricht-Lundborg type: a clinical and molecular genetic study of a family from the United States with four affected sibs. Neurology. 1993 Nov;43(11):2384–2386. doi: 10.1212/wnl.43.11.2384. [DOI] [PubMed] [Google Scholar]
- Lehesjoki A. E., Koskiniemi M., Norio R., Tirrito S., Sistonen P., Lander E., de la Chapelle A. Localization of the EPM1 gene for progressive myoclonus epilepsy on chromosome 21: linkage disequilibrium allows high resolution mapping. Hum Mol Genet. 1993 Aug;2(8):1229–1234. doi: 10.1093/hmg/2.8.1229. [DOI] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Malafosse A., Lehesjoki A. E., Genton P., Labauge P., Durand G., Tassinari C. A., Dravet C., Michelucci R., de la Chapelle A. Identical genetic locus for Baltic and Mediterranean myoclonus. Lancet. 1992 May 2;339(8801):1080–1081. doi: 10.1016/0140-6736(92)90667-r. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Puranam R. S. Epilepsy. Protease inhibitor implicated. Nature. 1996 May 2;381(6577):26–27. doi: 10.1038/381026a0. [DOI] [PubMed] [Google Scholar]
- Norio R., Koskiniemi M. Progressive myoclonus epilepsy: genetic and nosological aspects with special reference to 107 Finnish patients. Clin Genet. 1979 May;15(5):382–398. doi: 10.1111/j.1399-0004.1979.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Pennacchio L. A., Lehesjoki A. E., Stone N. E., Willour V. L., Virtaneva K., Miao J., D'Amato E., Ramirez L., Faham M., Koskiniemi M. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1) Science. 1996 Mar 22;271(5256):1731–1734. doi: 10.1126/science.271.5256.1731. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Evolution of proteins of the cystatin superfamily. J Mol Evol. 1990 Jan;30(1):60–71. doi: 10.1007/BF02102453. [DOI] [PubMed] [Google Scholar]
- Stone N. E., Fan J. B., Willour V., Pennacchio L. A., Warrington J. A., Hu A., de la Chapelle A., Lehesjoki A. E., Cox D. R., Myers R. M. Construction of a 750-kb bacterial clone contig and restriction map in the region of human chromosome 21 containing the progressive myoclonus epilepsy gene. Genome Res. 1996 Mar;6(3):218–225. doi: 10.1101/gr.6.3.218. [DOI] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele U., Assfalg-Machleidt I., Machleidt W., Auerswald E. A. N-terminal variants of recombinant stefin B: effect on affinity for papain and cathepsin B. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):125–136. [PubMed] [Google Scholar]
- Turk V., Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991 Jul 22;285(2):213–219. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- Virtaneva K., Miao J., Träskelin A. L., Stone N., Warrington J. A., Weissenbach J., Myers R. M., Cox D. R., Sistonen P., de la Chapelle A. Progressive myoclonus epilepsy EPM1 locus maps to a 175-kb interval in distal 21q. Am J Hum Genet. 1996 Jun;58(6):1247–1253. [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K., Mitchell S., Hubert R., Chen X. N., Colbern S., Huo Y. K., Gadomski C., Kim U. J., Korenberg J. R. Isolation and characterization of a candidate gene for progressive myoclonus epilepsy on 21q22.3. Hum Mol Genet. 1995 Apr;4(4):709–716. doi: 10.1093/hmg/4.4.709. [DOI] [PubMed] [Google Scholar]