Abstract
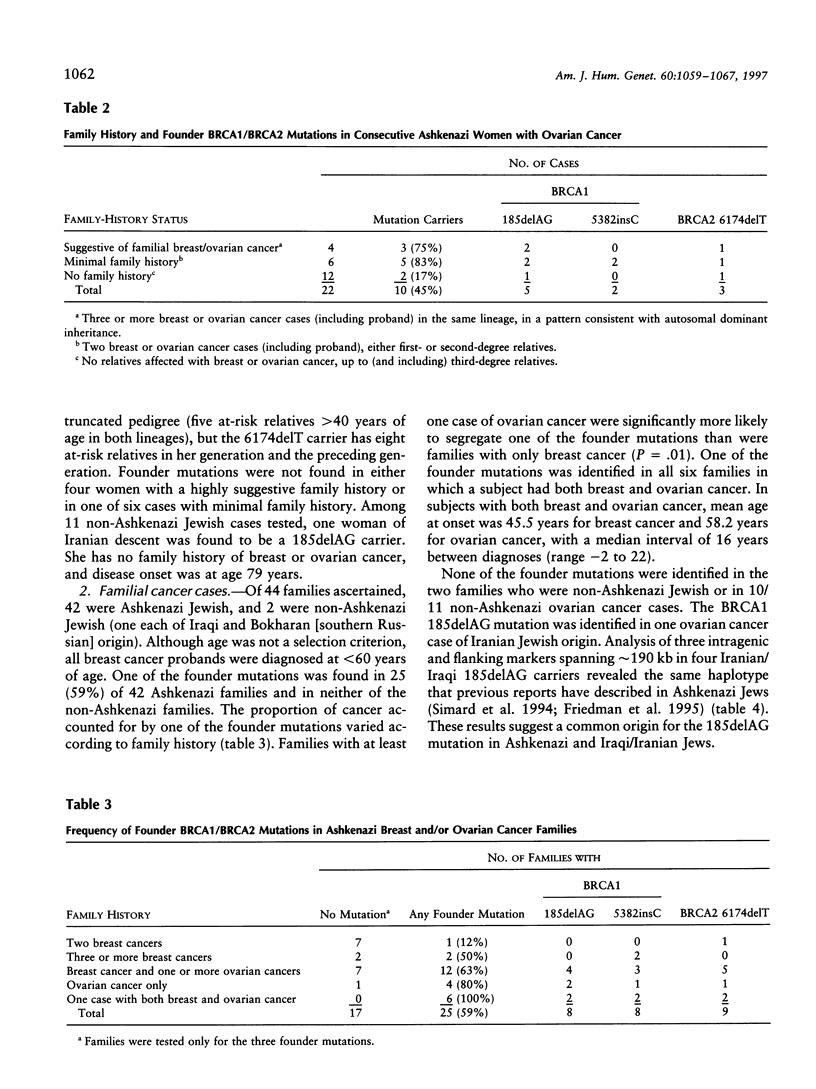
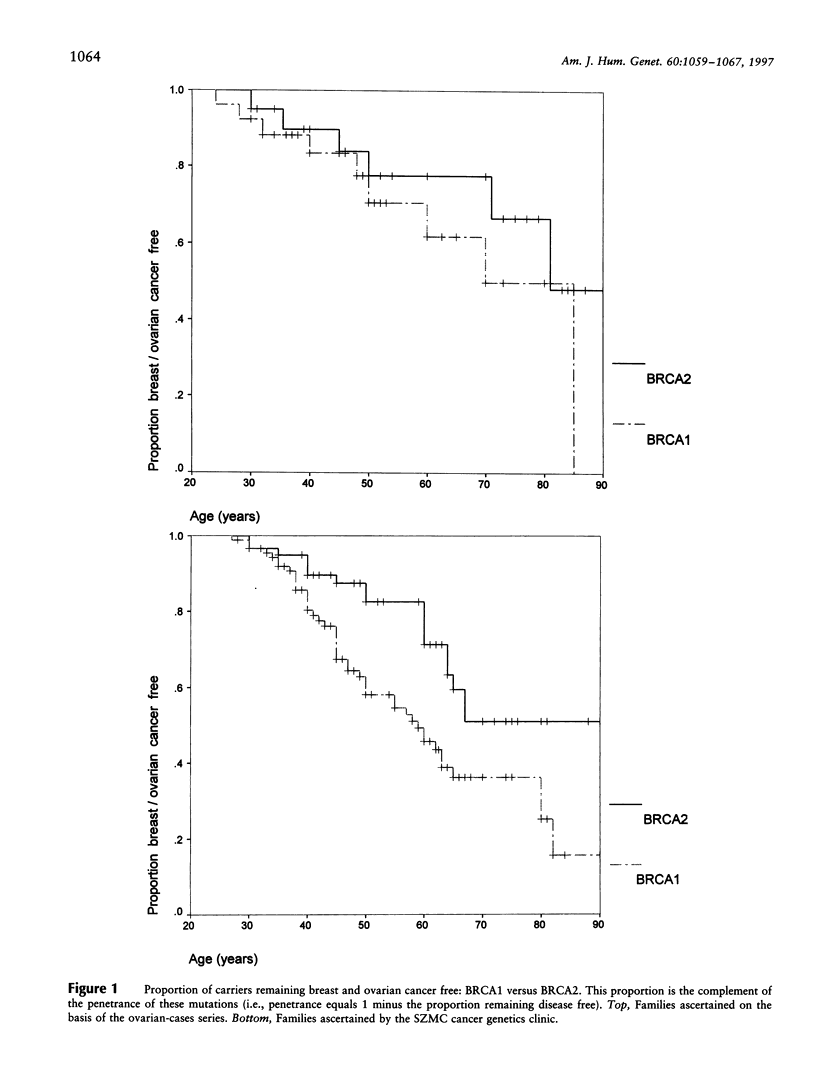
Germ-line BRCA1 and BRCA2 mutations account for most of familial breast-ovarian cancer. In Ashkenazi Jews, there is a high population frequency (approximately 2%) of three founder mutations: BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT. This study examined the frequency of these mutations in a series of Ashkenazi women with ovarian cancer unselected for family history, compared with the frequency of these mutations in families ascertained on the basis of family history of at least two affected women. Penetrance was compared, both according to the method of family ascertainment (i.e., on the basis of an unselected ovarian cancer proband vs. on the basis of family history) and for the BRCA1 founder mutations compared with the BRCA2 6174delT mutation. There was a high frequency (10/22; [45%]) of germ-line mutations in Ashkenazi women with ovarian cancer, even in those with minimal or no family history (7/18 [39%]). In high-risk Ashkenazi families, a founder mutation was found in 59% (25/42). Families with any case of ovarian cancer were significantly more likely to segregate a founder mutation than were families with site-specific breast cancer. Penetrance was higher in families ascertained on the basis of family history than in families ascertained on the basis of an unselected proband, but this difference was not significant. Penetrance of BRCA1 185delAG and BRCA1 5382insC was significantly higher than penetrance of BRCA2 6174delT (hazard ratio 2.1 [95% CI 1.2-3.8]; two-tailed P = .01). Thus, the high rate of germ-line BRCA1/BRCA2 mutations in Ashkenazi women and families with ovarian cancer is coupled with penetrance that is lower than previously estimated. This has been shown specifically for the BRCA2 6174delT mutation, but, because of ascertainment bias, it also may be true for BRCA1 mutations.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claus E. B., Risch N., Thompson W. D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991 Feb;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- Couch F. J., Farid L. M., DeShano M. L., Tavtigian S. V., Calzone K., Campeau L., Peng Y., Bogden B., Chen Q., Neuhausen S. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996 May;13(1):123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- Couch F. J., Weber B. L. Mutations and polymorphisms in the familial early-onset breast cancer (BRCA1) gene. Breast Cancer Information Core. Hum Mutat. 1996;8(1):8–18. doi: 10.1002/humu.1380080102. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Szabo C. I., Ostermeyer E. A., Dowd P., Butler L., Park T., Lee M. K., Goode E. L., Rowell S. E., King M. C. Novel inherited mutations and variable expressivity of BRCA1 alleles, including the founder mutation 185delAG in Ashkenazi Jewish families. Am J Hum Genet. 1995 Dec;57(6):1284–1297. [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Rock M. J., Prockop D. J. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10325–10329. doi: 10.1073/pnas.90.21.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayther S. A., Warren W., Mazoyer S., Russell P. A., Harrington P. A., Chiano M., Seal S., Hamoudi R., van Rensburg E. J., Dunning A. M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995 Dec;11(4):428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Johannesdottir G., Gudmundsson J., Bergthorsson J. T., Arason A., Agnarsson B. A., Eiriksdottir G., Johannsson O. T., Borg A., Ingvarsson S., Easton D. F. High prevalence of the 999del5 mutation in icelandic breast and ovarian cancer patients. Cancer Res. 1996 Aug 15;56(16):3663–3665. [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Modan B., Gak E., Sade-Bruchim R. B., Hirsh-Yechezkel G., Theodor L., Lubin F., Ben-Baruch G., Beller U., Fishman A., Dgani R. High frequency of BRCA1 185delAG mutation in ovarian cancer in Israel. National Israel Study of Ovarian Cancer. JAMA. 1996 Dec 11;276(22):1823–1825. [PubMed] [Google Scholar]
- NEWILL V. A. Distribution of cancer mortality among etimic subgroups of the white population of New York City, 1953-58. J Natl Cancer Inst. 1961 Feb;26:405–417. [PubMed] [Google Scholar]
- Neuhausen S., Gilewski T., Norton L., Tran T., McGuire P., Swensen J., Hampel H., Borgen P., Brown K., Skolnick M. Recurrent BRCA2 6174delT mutations in Ashkenazi Jewish women affected by breast cancer. Nat Genet. 1996 May;13(1):126–128. doi: 10.1038/ng0596-126. [DOI] [PubMed] [Google Scholar]
- Novakovic B., Goldstein A. M., Tucker M. A. Validation of family history of cancer in deceased family members. J Natl Cancer Inst. 1996 Oct 16;88(20):1492–1493. doi: 10.1093/jnci/88.20.1492. [DOI] [PubMed] [Google Scholar]
- Oddoux C., Struewing J. P., Clayton C. M., Neuhausen S., Brody L. C., Kaback M., Haas B., Norton L., Borgen P., Jhanwar S. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet. 1996 Oct;14(2):188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- Offit K., Gilewski T., McGuire P., Schluger A., Hampel H., Brown K., Swensen J., Neuhausen S., Skolnick M., Norton L. Germline BRCA1 185delAG mutations in Jewish women with breast cancer. Lancet. 1996 Jun 15;347(9016):1643–1645. doi: 10.1016/s0140-6736(96)91484-1. [DOI] [PubMed] [Google Scholar]
- Roa B. B., Boyd A. A., Volcik K., Richards C. S. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996 Oct;14(2):185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- Sher C., Sharabini-Gargir L., Shohat M. Breast cancer and BRCA1 mutations. N Engl J Med. 1996 May 2;334(18):1199–1200. [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Tonin P., Weber B., Offit K., Couch F., Rebbeck T. R., Neuhausen S., Godwin A. K., Daly M., Wagner-Costalos J., Berman D. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996 Nov;2(11):1179–1183. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]


