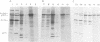Abstract
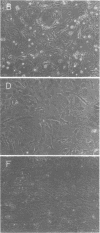
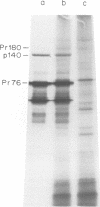
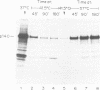
Several clones of Fujinami sarcoma virus (FSV) isolated from a laboratory stock or from mutagenized virus were temperature sensitive (ts) in transformation of cells in culture. When shifted from the permissive (37°C) to the nonpermissive (41.5°C) temperature, the cellular phenotype reverted to normal within 2 h, but it required about 48 h at 37°C to revert back to the transformed morphology. A temperature-resistant (tr) FSV clone was isolated from a tumor of an animal. All ts mutants were tumorigenic in animals but induced tumors only after latent periods of 12 to 25 days, compared to 5 to 6 days with tr virus. The ts lesions of the FSV mutants affected 90% of the phosphorylation of the nonstructural, gag-related 140,000-kilodalton phosphoprotein coded by FSV (p140), but did not affect virus replication or the synthesis of p140. Upon shifting from the permissive to the nonpermissive temperature, p140 was 90% dephosphorylated with an approximate 32P half-life of 20 min. When shifted back to the permissive temperature, the preexisting p140 was rephosphorylated in the absence of protein synthesis within a 90-min test period. Likewise, most of the phosphate of fully phosphorylated p140 was exchanged at the permissive temperature within 30 to 90 min even when protein synthesis was inhibited. However, the protein structure of p140 had a half-life of 5 h at both temperatures. These results prove p140 to be a substrate of reversible phosphorylation. Superinfection and transformation of ts FSV-infected cells maintained at the nonpermissive temperature with acute leukemia virus MC29 failed to phosphorylate p140. It would follow that in vivo phosphorylation of ts p140 is controlled by an FSV-specific mechanism and is a prerequisite, not a consequence, of transformation. p140 of ts FSV recovered from cells maintained at 41.5°C with anti-gag serum was over 10 times less phosphorylated by associated kinase than the same protein recovered from cells at 37°C if assayed in vitro at 20°C. This kinase activity associated with or dissociated from p140 with a half-life of less than 30 min during temperature shifts of ts FSV-infected cells. However, p140 recovered from ts FSV-infected cells maintained at 37°C was phosphorylated by associated kinase in vitro not only at 20°C but also, and essentially at the same level, at 41.5°C. This suggests that the kinase associated with the immunocomplex of p140 of ts FSV is not temperature sensitive. p140 translated in vitro from ts and tr FSV RNA lacked kinase activity. We conclude that a fully phosphorylated p140 is necessary for the maintenance of transformation by FSV. This is consistent with the notion that other highly oncogenic viruses also code for nonstructural phosphoproteins with probable transforming function. A model which postulates that p140 is a substrate of reversible phosphorylation and that the lesion of the ts FSV clones described herein affects association of p140 with a cellular kinase rather than a hypothetical intrinsic kinase activity of the protein is most compatible with our data.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M., Courtneidge S. A., Levinson A. D., Oppermann H., Quintrell N., Sheiness D. K., Weiss S. R., Varmus H. E. Origin and function of avian retrovirus transforming genes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):919–930. doi: 10.1101/sqb.1980.044.01.099. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
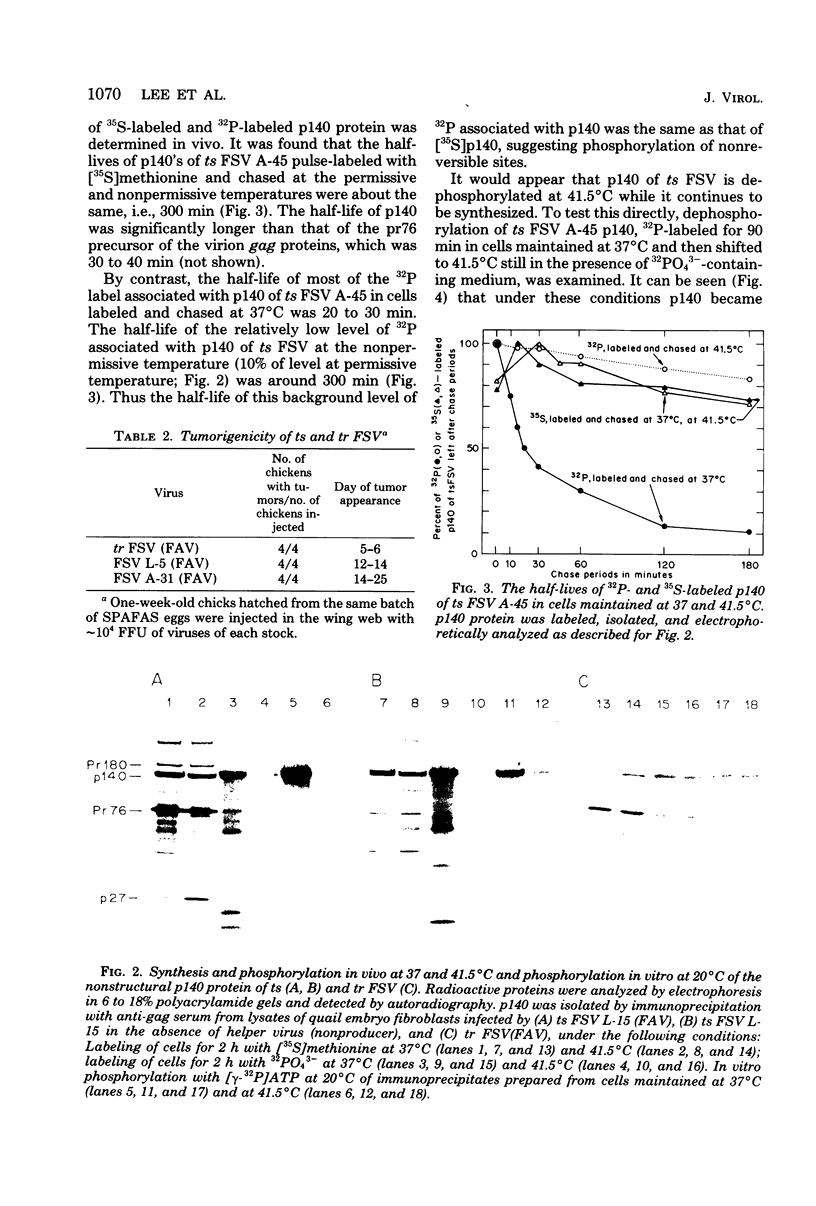
- Bister K., Lee W. H., Duesberg P. H. Phosphorylation of the nonstructural proteins encoded by three avian acute leukemia viruses and by avian fujinami sarcoma virus. J Virol. 1980 Nov;36(2):617–621. doi: 10.1128/jvi.36.2.617-621.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G., Hayman M. J., Duesberg P. H. OK10, an avian acute leukemia virus of the MC 29 subgroup with a unique genetic structure. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7142–7146. doi: 10.1073/pnas.77.12.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Erikson R. I., Collett M. S., Erikson E., Purchio A. F., Brugge J. S. Protein phosphorylation mediated by partially purified avian sarcoma virus transforming-gene product. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):907–917. doi: 10.1101/sqb.1980.044.01.098. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
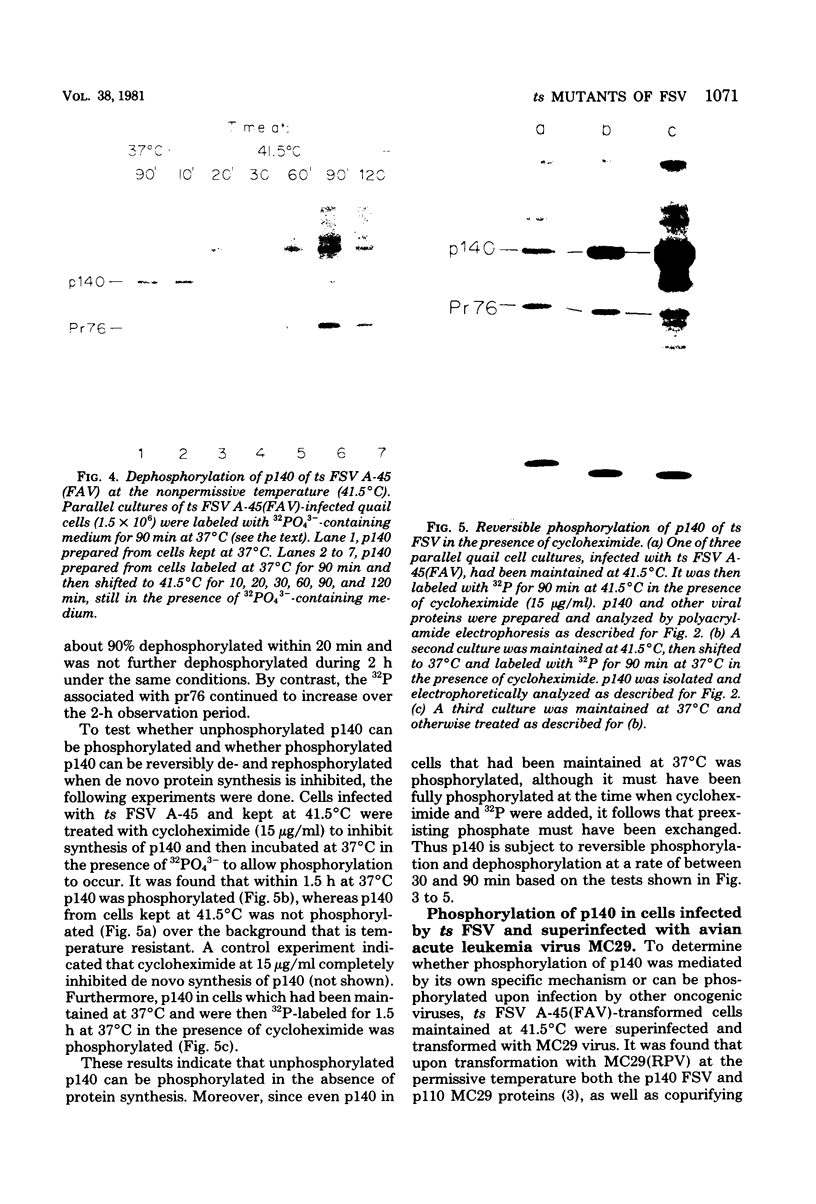
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Richert N. D., Davies P. J., Jay G., Pastan I. H. Characterization of an immune complex kinase in immunoprecipitates of avian sarcoma virus-transformed fibroblasts. J Virol. 1979 Sep;31(3):696–706. doi: 10.1128/jvi.31.3.696-706.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]