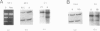Abstract
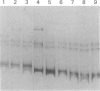
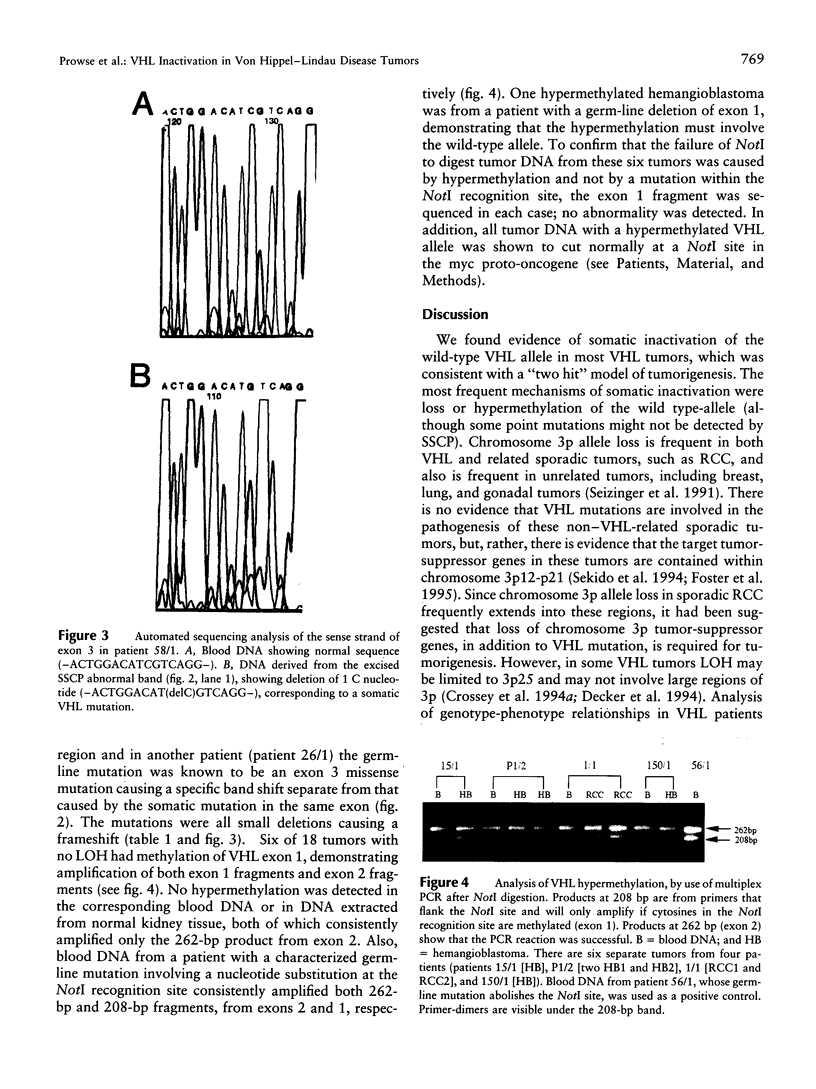
Von Hippel-Lindau (VHL) disease is a dominantly inherited disorder predisposing to retinal and CNS hemangioblastomas, renal cell carcinoma (RCC), pheochromocytoma, and pancreatic tumors. Interfamilial differences in predisposition to pheochromocytoma reflect allelic heterogeneity such that there is a strong association between missense mutations and risk of pheochromocytoma. We investigated the mechanism of tumorigenesis in VHL disease tumors to determine whether there were differences between tumor types or classes of germ-line mutations. Fifty-three tumors (30 RCCs, 15 hemangioblastomas, 5 pheochromocytomas, and 3 pancreatic tumors) from 33 patients (27 kindreds) with VHL disease were analyzed. Overall, 51% of 45 informative tumors showed loss of heterozygosity (LOH) at the VHL locus. In 11 cases it was possible to distinguish between loss of the wild-type and mutant alleles, and in each case the wild-type allele was lost. LOH was detected in all tumor types and occurred in the presence of both germ-line missense mutations and other types of germline mutation associated with a low risk of pheochromocytoma. Intragenic somatic mutations were detected in three tumors (all hemangioblastomas) and in two of these could be shown to occur in the wild-type allele. This provides the first example of homozygous inactivation of the VHL by small intragenic mutations in this type of tumor. Hypermethylation of the VHL gene was detected in 33% (6/18) of tumors without LOH, including 2 RCCs and 4 hemangioblastomas. Although hypermethylation of the VHL gene has been reported previously in nonfamilial RCC and although methylation of tumor-suppressor genes has been implicated in the pathogenesis of other sporadic cancers, this is the first report of somatic methylation in a familial cancer syndrome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Lane W. S., Conaway J. W., Conaway R. C. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science. 1995 Sep 8;269(5229):1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- Brauch H., Kishida T., Glavac D., Chen F., Pausch F., Höfler H., Latif F., Lerman M. I., Zbar B., Neumann H. P. Von Hippel-Lindau (VHL) disease with pheochromocytoma in the Black Forest region of Germany: evidence for a founder effect. Hum Genet. 1995 May;95(5):551–556. doi: 10.1007/BF00223868. [DOI] [PubMed] [Google Scholar]
- Chen F., Kishida T., Yao M., Hustad T., Glavac D., Dean M., Gnarra J. R., Orcutt M. L., Duh F. M., Glenn G. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat. 1995;5(1):66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- Crossey P. A., Eng C., Ginalska-Malinowska M., Lennard T. W., Wheeler D. C., Ponder B. A., Maher E. R. Molecular genetic diagnosis of von Hippel-Lindau disease in familial phaeochromocytoma. J Med Genet. 1995 Nov;32(11):885–886. doi: 10.1136/jmg.32.11.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossey P. A., Foster K., Richards F. M., Phipps M. E., Latif F., Tory K., Jones M. H., Bentley E., Kumar R., Lerman M. I. Molecular genetic investigations of the mechanism of tumourigenesis in von Hippel-Lindau disease: analysis of allele loss in VHL tumours. Hum Genet. 1994 Jan;93(1):53–58. doi: 10.1007/BF00218913. [DOI] [PubMed] [Google Scholar]
- Crossey P. A., Richards F. M., Foster K., Green J. S., Prowse A., Latif F., Lerman M. I., Zbar B., Affara N. A., Ferguson-Smith M. A. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet. 1994 Aug;3(8):1303–1308. doi: 10.1093/hmg/3.8.1303. [DOI] [PubMed] [Google Scholar]
- Decker H. J., Klauck S. M., Lawrence J. B., McNeil J., Smith D., Gemmill R. M., Sandberg A. A., Neumann H. H., Simon B., Green J. Cytogenetic and fluorescence in situ hybridization studies on sporadic and hereditary tumors associated with von Hippel-Lindau syndrome (VHL). Cancer Genet Cytogenet. 1994 Oct;77(1):1–13. doi: 10.1016/0165-4608(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Duan D. R., Pause A., Burgess W. H., Aso T., Chen D. Y., Garrett K. P., Conaway R. C., Conaway J. W., Linehan W. M., Klausner R. D. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995 Sep 8;269(5229):1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- Eng C., Crossey P. A., Mulligan L. M., Healey C. S., Houghton C., Prowse A., Chew S. L., Dahia P. L., O'Riordan J. L., Toledo S. P. Mutations in the RET proto-oncogene and the von Hippel-Lindau disease tumour suppressor gene in sporadic and syndromic phaeochromocytomas. J Med Genet. 1995 Dec;32(12):934–937. doi: 10.1136/jmg.32.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K., Prowse A., van den Berg A., Fleming S., Hulsbeek M. M., Crossey P. A., Richards F. M., Cairns P., Affara N. A., Ferguson-Smith M. A. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994 Dec;3(12):2169–2173. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- Gnarra J. R., Tory K., Weng Y., Schmidt L., Wei M. H., Li H., Latif F., Liu S., Chen F., Duh F. M. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994 May;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H., Kondo K., Ito S., Yamamoto I., Fujii S., Torigoe S., Sakai N., Hosaka M., Shuin T., Yao M. Somatic mutations of the von Hippel-Lindau tumor suppressor gene in sporadic central nervous system hemangioblastomas. Cancer Res. 1994 Sep 15;54(18):4845–4847. [PubMed] [Google Scholar]
- Kibel A., Iliopoulos O., DeCaprio J. A., Kaelin W. G., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995 Sep 8;269(5229):1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Jaenisch R. DNA methylation and cancer. Hum Mol Genet. 1994;3(Spec No):1487–1495. doi: 10.1093/hmg/3.suppl_1.1487. [DOI] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Maher E. R., Webster A. R., Richards F. M., Green J. S., Crossey P. A., Payne S. J., Moore A. T. Phenotypic expression in von Hippel-Lindau disease: correlations with germline VHL gene mutations. J Med Genet. 1996 Apr;33(4):328–332. doi: 10.1136/jmg.33.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E. R., Yates J. R., Harries R., Benjamin C., Harris R., Moore A. T., Ferguson-Smith M. A. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990 Nov;77(283):1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Neumann H. P., Eng C., Mulligan L. M., Glavac D., Zäuner I., Ponder B. A., Crossey P. A., Maher E. R., Brauch H. Consequences of direct genetic testing for germline mutations in the clinical management of families with multiple endocrine neoplasia, type II. JAMA. 1995 Oct 11;274(14):1149–1151. [PubMed] [Google Scholar]
- Payne S. J., Richards F. M., Maher E. R. A PCR generated AccI RFLP in the 3' untranslated region of the von Hippel-Lindau disease (VHL) tumour suppressor gene. Hum Mol Genet. 1994 Feb;3(2):390–390. doi: 10.1093/hmg/3.2.390. [DOI] [PubMed] [Google Scholar]
- Sekido Y., Bader S., Latif F., Gnarra J. R., Gazdar A. F., Linehan W. M., Zbar B., Lerman M. I., Minna J. D. Molecular analysis of the von Hippel-Lindau disease tumor suppressor gene in human lung cancer cell lines. Oncogene. 1994 Jun;9(6):1599–1604. [PubMed] [Google Scholar]
- Shuin T., Kondo K., Torigoe S., Kishida T., Kubota Y., Hosaka M., Nagashima Y., Kitamura H., Latif F., Zbar B. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994 Jun 1;54(11):2852–2855. [PubMed] [Google Scholar]