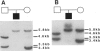Abstract
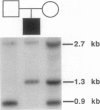
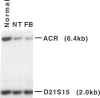
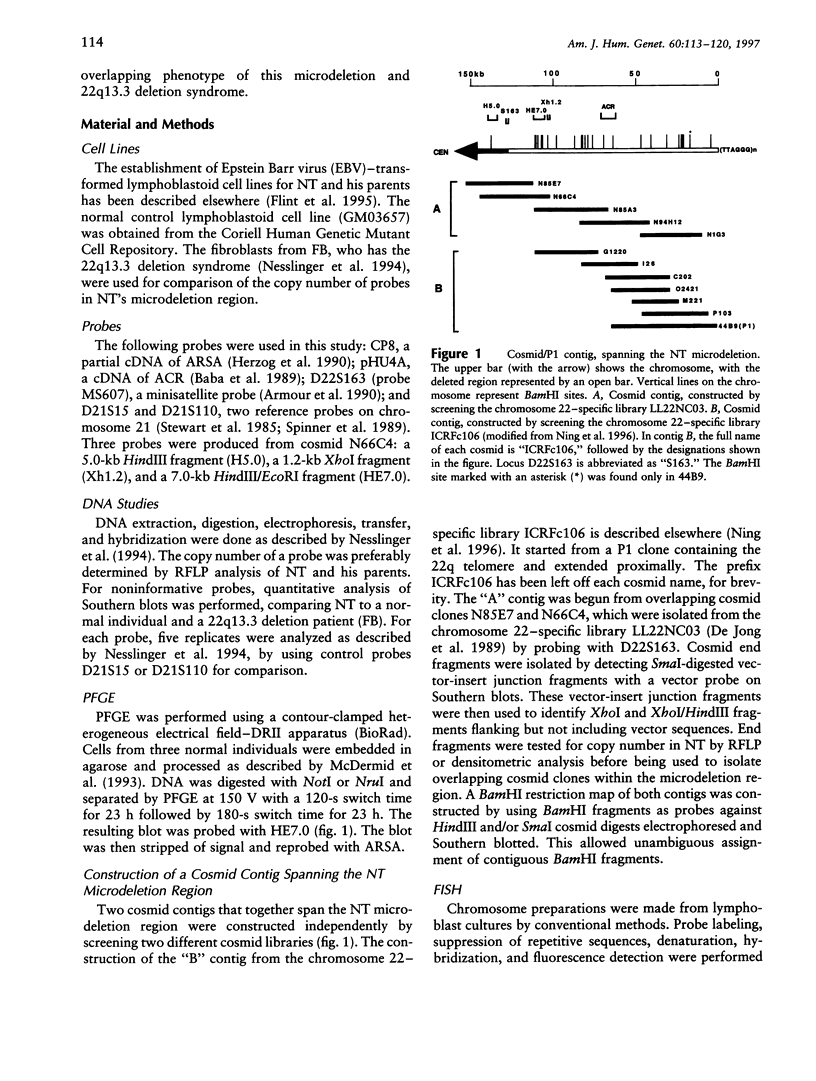
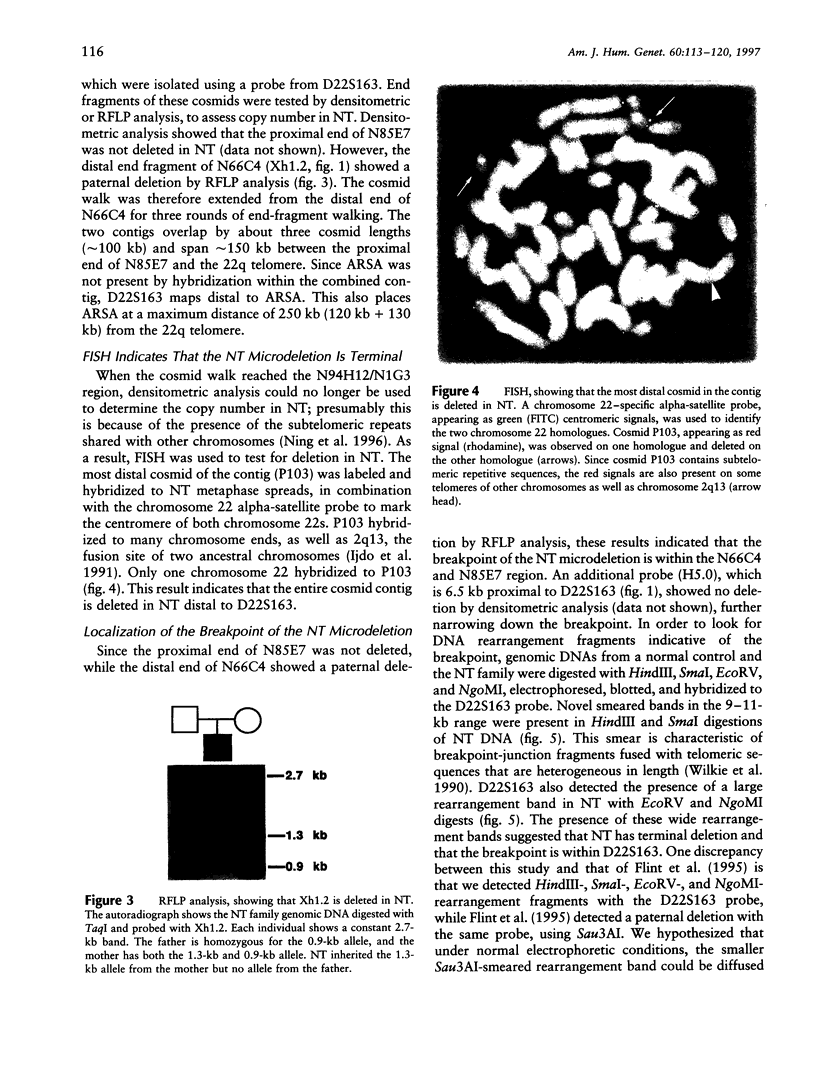
We have analyzed a recently described 22q13.3 microdeletion in a child with some overlapping features of the cytologically visible 22q13.3 deletion syndrome. Patient NT, who shows mild mental retardation and delay of expressive speech, was previously found to have a paternal microdeletion in the subtelomeric region of 22q. In order to characterize this abnormality further, we have constructed a cosmid/P1 contig covering the terminal 150 kb of 22q, which encompasses the 130-kb microdeletion. The microdeletion breakpoint is within the VNTR locus D22S163. The cloning of the breakpoint sequence revealed that the broken chromosome end was healed by the addition of telomeric repeats, indicating that the microdeletion is terminal. This is the first cloned terminal deletion breakpoint on a human chromosome other than 16p. The cosmid/P1 contig was mapped by pulsed-field gel electrophoresis analysis to within 120 kb of the arylsulfatase A gene, which places the contig in relation to genetic and physical maps of the chromosome. The acrosin gene maps within the microdeletion, approximately 70 kb from the telomere. With the distal end of chromosome 22q cloned, it is now possible to isolate genes that may be involved in the overlapping phenotype of this microdeletion and 22q13.3 deletion syndrome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Jeffreys A. J. STS for minisatellite MS607 (D22S163). Nucleic Acids Res. 1991 Jun 11;19(11):3158–3158. doi: 10.1093/nar/19.11.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour J. A., Povey S., Jeremiah S., Jeffreys A. J. Systematic cloning of human minisatellites from ordered array charomid libraries. Genomics. 1990 Nov;8(3):501–512. doi: 10.1016/0888-7543(90)90037-u. [DOI] [PubMed] [Google Scholar]
- Baba T., Watanabe K., Kashiwabara S., Arai Y. Primary structure of human proacrosin deduced from its cDNA sequence. FEBS Lett. 1989 Feb 27;244(2):296–300. doi: 10.1016/0014-5793(89)80549-6. [DOI] [PubMed] [Google Scholar]
- Collins J. E., Cole C. G., Smink L. J., Garrett C. L., Leversha M. A., Soderlund C. A., Maslen G. L., Everett L. A., Rice K. M., Coffey A. J. A high-density YAC contig map of human chromosome 22. Nature. 1995 Sep 28;377(6547 Suppl):367–379. doi: 10.1038/377367a0. [DOI] [PubMed] [Google Scholar]
- Dumanski J. P., Carlbom E., Collins V. P., Nordenskjöld M., Emanuel B. S., Budarf M. L., McDermid H. E., Wolff R., O'Connell P., White R. A map of 22 loci on human chromosome 22. Genomics. 1991 Nov;11(3):709–719. doi: 10.1016/0888-7543(91)90079-t. [DOI] [PubMed] [Google Scholar]
- Fields C., Adams M. D., White O., Venter J. C. How many genes in the human genome? Nat Genet. 1994 Jul;7(3):345–346. doi: 10.1038/ng0794-345. [DOI] [PubMed] [Google Scholar]
- Flint J., Craddock C. F., Villegas A., Bentley D. P., Williams H. J., Galanello R., Cao A., Wood W. G., Ayyub H., Higgs D. R. Healing of broken human chromosomes by the addition of telomeric repeats. Am J Hum Genet. 1994 Sep;55(3):505–512. [PMC free article] [PubMed] [Google Scholar]
- Flint J., Wilkie A. O., Buckle V. J., Winter R. M., Holland A. J., McDermid H. E. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat Genet. 1995 Feb;9(2):132–140. doi: 10.1038/ng0295-132. [DOI] [PubMed] [Google Scholar]
- Greger V., Woolf E., Lalande M. Cloning of the breakpoints of a submicroscopic deletion in an Angelman syndrome patient. Hum Mol Genet. 1993 Jul;2(7):921–924. doi: 10.1093/hmg/2.7.921. [DOI] [PubMed] [Google Scholar]
- Herzog R., Holzmann K., Blin N. A TaqI RFLP for the human arylsulfatase A gene. Nucleic Acids Res. 1990 Nov 25;18(22):6746–6746. doi: 10.1093/nar/18.22.6746-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber I., Bitner-Glindzicz M., de Kok Y. J., van der Maarel S. M., Ishikawa-Brush Y., Monaco A. P., Robinson D., Malcolm S., Pembrey M. E., Brunner H. G. X-linked mixed deafness (DFN3): cloning and characterization of the critical region allows the identification of novel microdeletions. Hum Mol Genet. 1994 Jul;3(7):1151–1154. doi: 10.1093/hmg/3.7.1151. [DOI] [PubMed] [Google Scholar]
- IJdo J. W., Baldini A., Ward D. C., Reeders S. T., Wells R. A. Origin of human chromosome 2: an ancestral telomere-telomere fusion. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9051–9055. doi: 10.1073/pnas.88.20.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm U., Müller-Esterl W., Engel W. Acrosin, the peculiar sperm-specific serine protease. Hum Genet. 1991 Oct;87(6):635–641. doi: 10.1007/BF00201716. [DOI] [PubMed] [Google Scholar]
- Koukoulis G. N., Vantman D., Dennison L., Banks S. M., Sherins R. J. Low acrosin activity in a subgroup of men with idiopathic infertility dose not correlate with sperm density, percent motility, curvilinear velocity, or linearity. Fertil Steril. 1989 Jul;52(1):120–127. doi: 10.1016/s0015-0282(16)60800-2. [DOI] [PubMed] [Google Scholar]
- Kuwano A., Ledbetter S. A., Dobyns W. B., Emanuel B. S., Ledbetter D. H. Detection of deletions and cryptic translocations in Miller-Dieker syndrome by in situ hybridization. Am J Hum Genet. 1991 Oct;49(4):707–714. [PMC free article] [PubMed] [Google Scholar]
- Lamb J., Harris P. C., Wilkie A. O., Wood W. G., Dauwerse J. G., Higgs D. R. De novo truncation of chromosome 16p and healing with (TTAGGG)n in the alpha-thalassemia/mental retardation syndrome (ATR-16). Am J Hum Genet. 1993 Apr;52(4):668–676. [PMC free article] [PubMed] [Google Scholar]
- Levy A., Demczuk S., Aurias A., Depétris D., Mattei M. G., Philip N. Interstitial 22q11 microdeletion excluding the ADU breakpoint in a patient with DiGeorge syndrome. Hum Mol Genet. 1995 Dec;4(12):2417–2419. doi: 10.1093/hmg/4.12.2417. [DOI] [PubMed] [Google Scholar]
- Liu D. Y., Baker H. W. Inhibition of acrosin activity with a trypsin inhibitor blocks human sperm penetration of the zona pellucida. Biol Reprod. 1993 Feb;48(2):340–348. doi: 10.1095/biolreprod48.2.340. [DOI] [PubMed] [Google Scholar]
- McDermid H. E., Budarf M. L., Emanuel B. S. Long-range restriction map of human chromosome 22q11-22q12 between the lambda immunoglobulin locus and the Ewing sarcoma breakpoint. Genomics. 1993 Nov;18(2):308–318. doi: 10.1006/geno.1993.1470. [DOI] [PubMed] [Google Scholar]
- Mohsenian M., Syner F. N., Moghissi K. S. A study of sperm acrosin in patients with unexplained infertility. Fertil Steril. 1982 Feb;37(2):223–229. [PubMed] [Google Scholar]
- Nesslinger N. J., Gorski J. L., Kurczynski T. W., Shapira S. K., Siegel-Bartelt J., Dumanski J. P., Cullen R. F., Jr, French B. N., McDermid H. E. Clinical, cytogenetic, and molecular characterization of seven patients with deletions of chromosome 22q13.3. Am J Hum Genet. 1994 Mar;54(3):464–472. [PMC free article] [PubMed] [Google Scholar]
- Ning Y., Rosenberg M., Biesecker L. G., Ledbetter D. H. Isolation of the human chromosome 22q telomere and its application to detection of cryptic chromosomal abnormalities. Hum Genet. 1996 Jun;97(6):765–769. doi: 10.1007/BF02346187. [DOI] [PubMed] [Google Scholar]
- Saccone S., De Sario A., Della Valle G., Bernardi G. The highest gene concentrations in the human genome are in telomeric bands of metaphase chromosomes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4913–4917. doi: 10.1073/pnas.89.11.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner N. B., Eunpu D. L., Schmickel R. D., Zackai E. H., McEldrew D., Bunin G. R., McDermid H., Emanuel B. S. The role of cytologic NOR variants in the etiology of trisomy 21. Am J Hum Genet. 1989 May;44(5):631–638. [PMC free article] [PubMed] [Google Scholar]
- Takano H., Yanagimachi R., Urch U. A. Evidence that acrosin activity is important for the development of fusibility of mammalian spermatozoa with the oolemma: inhibitor studies using the golden hamster. Zygote. 1993 Feb;1(1):79–91. doi: 10.1017/s0967199400001325. [DOI] [PubMed] [Google Scholar]
- Vazquez-Levin M. H., Reventos J., Gordon J. W. Molecular cloning, sequencing and restriction mapping of the genomic sequence encoding human proacrosin. Eur J Biochem. 1992 Jul 1;207(1):23–28. doi: 10.1111/j.1432-1033.1992.tb17014.x. [DOI] [PubMed] [Google Scholar]
- Weier H. U., Kleine H. D., Gray J. W. Labeling of the centromeric region on human chromosome 8 by in situ hybridization. Hum Genet. 1991 Aug;87(4):489–494. doi: 10.1007/BF00197174. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O., Lamb J., Harris P. C., Finney R. D., Higgs D. R. A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature. 1990 Aug 30;346(6287):868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]