Abstract
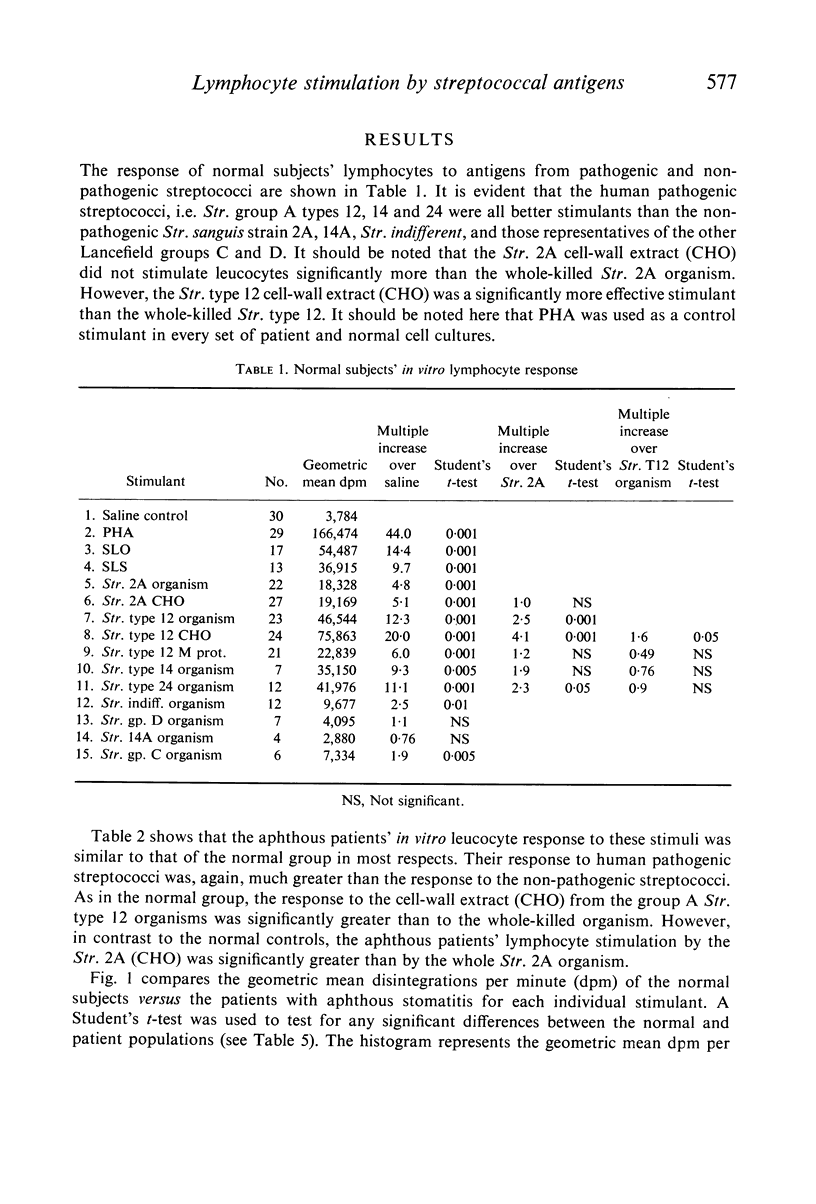
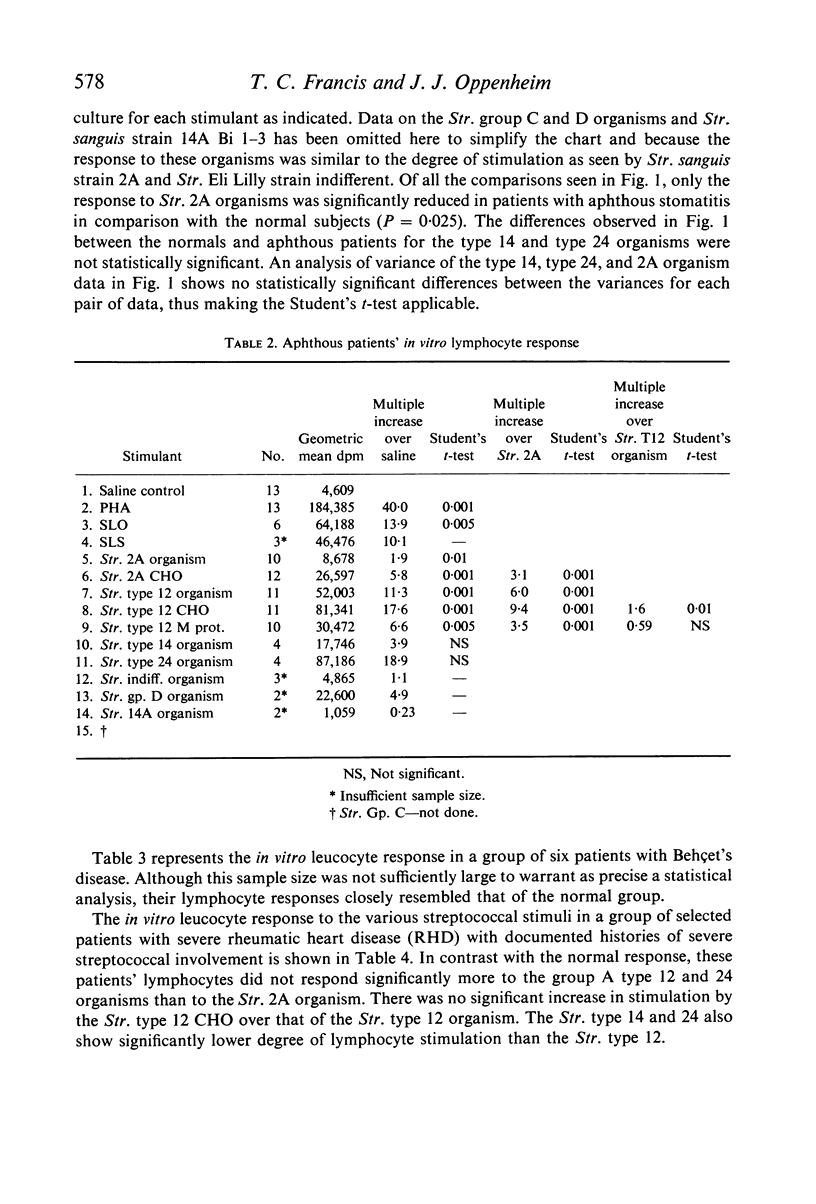
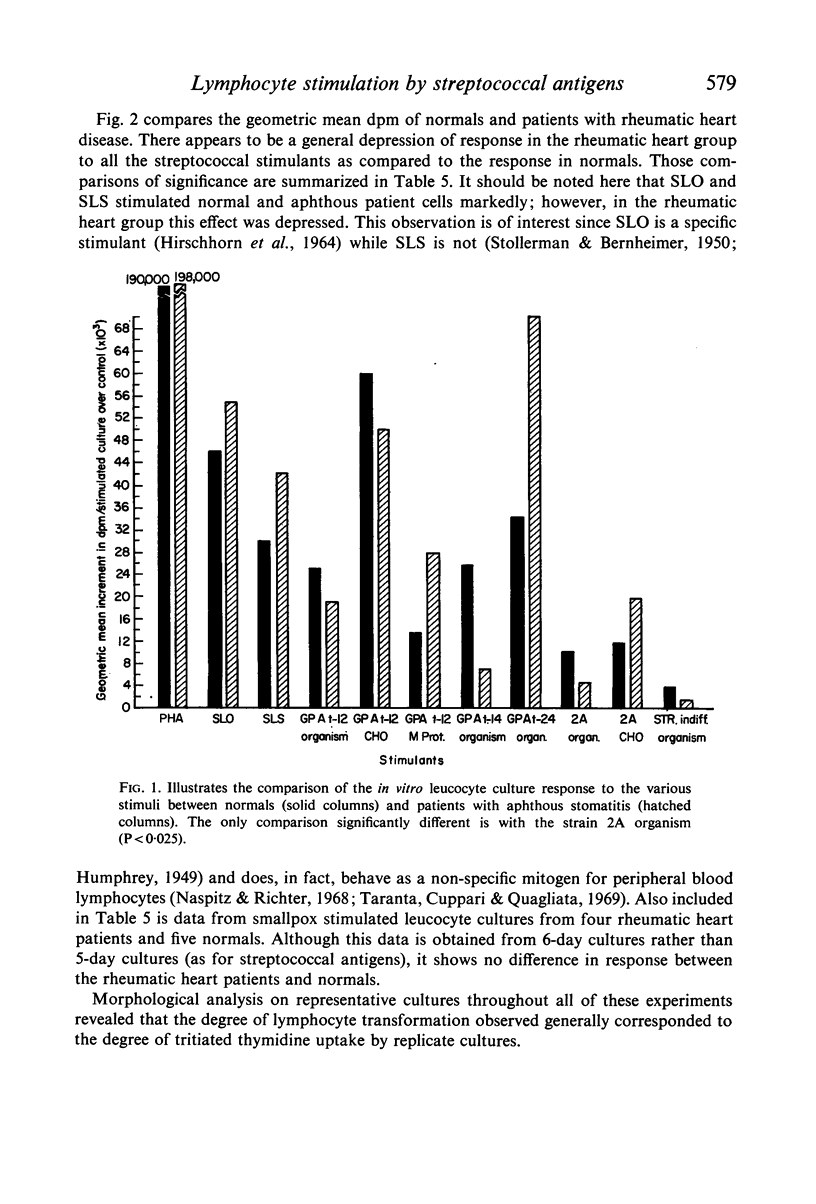
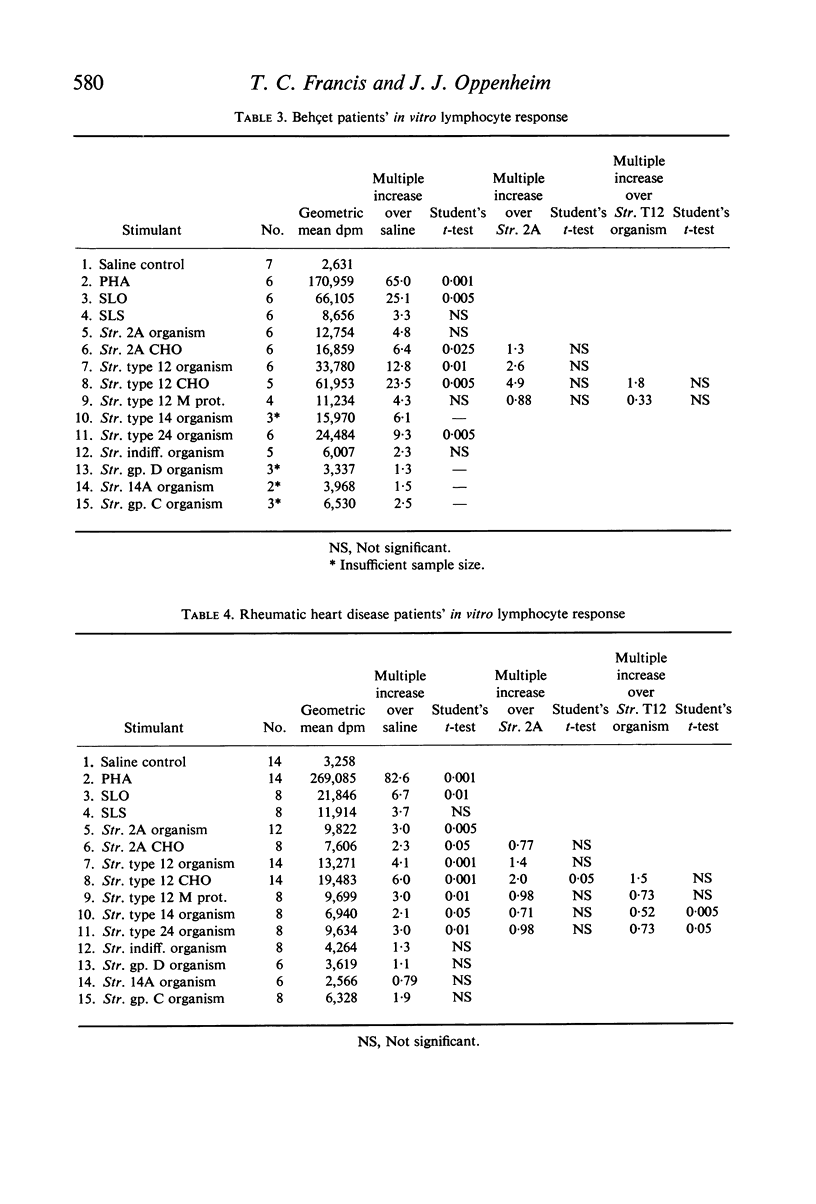
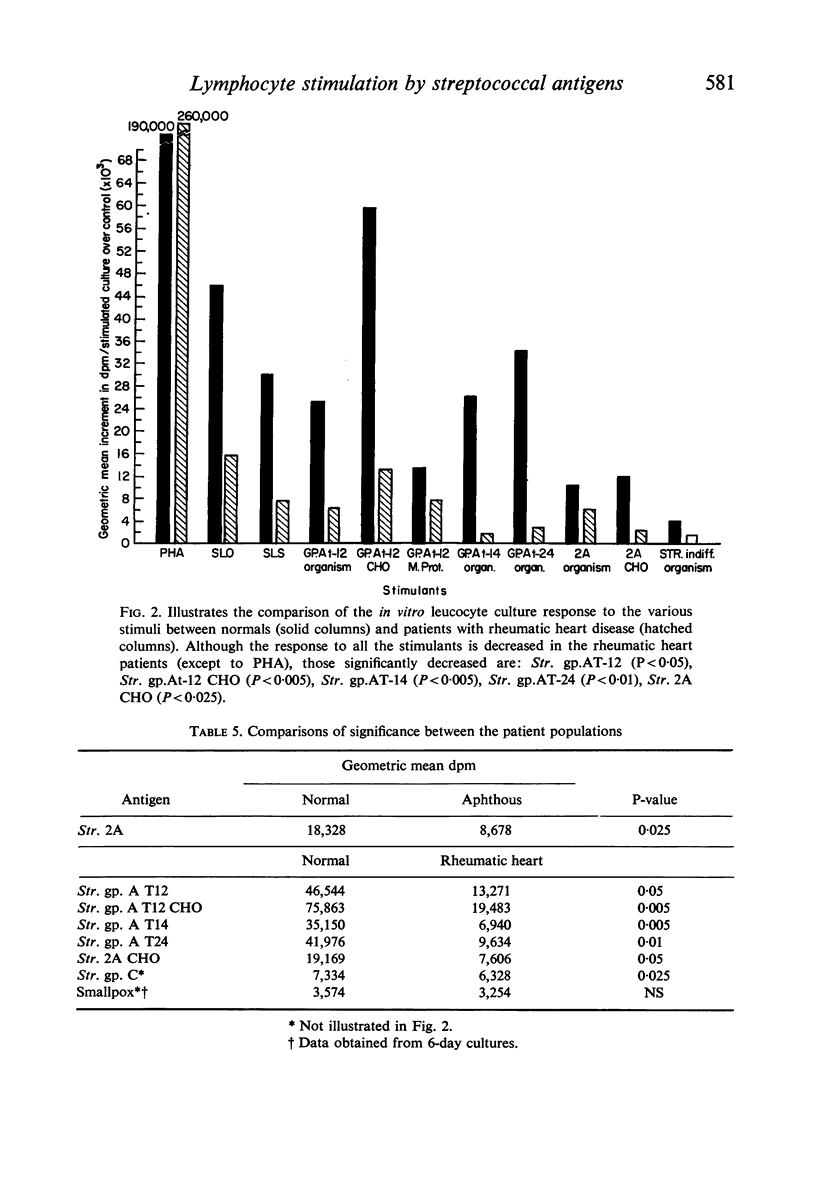
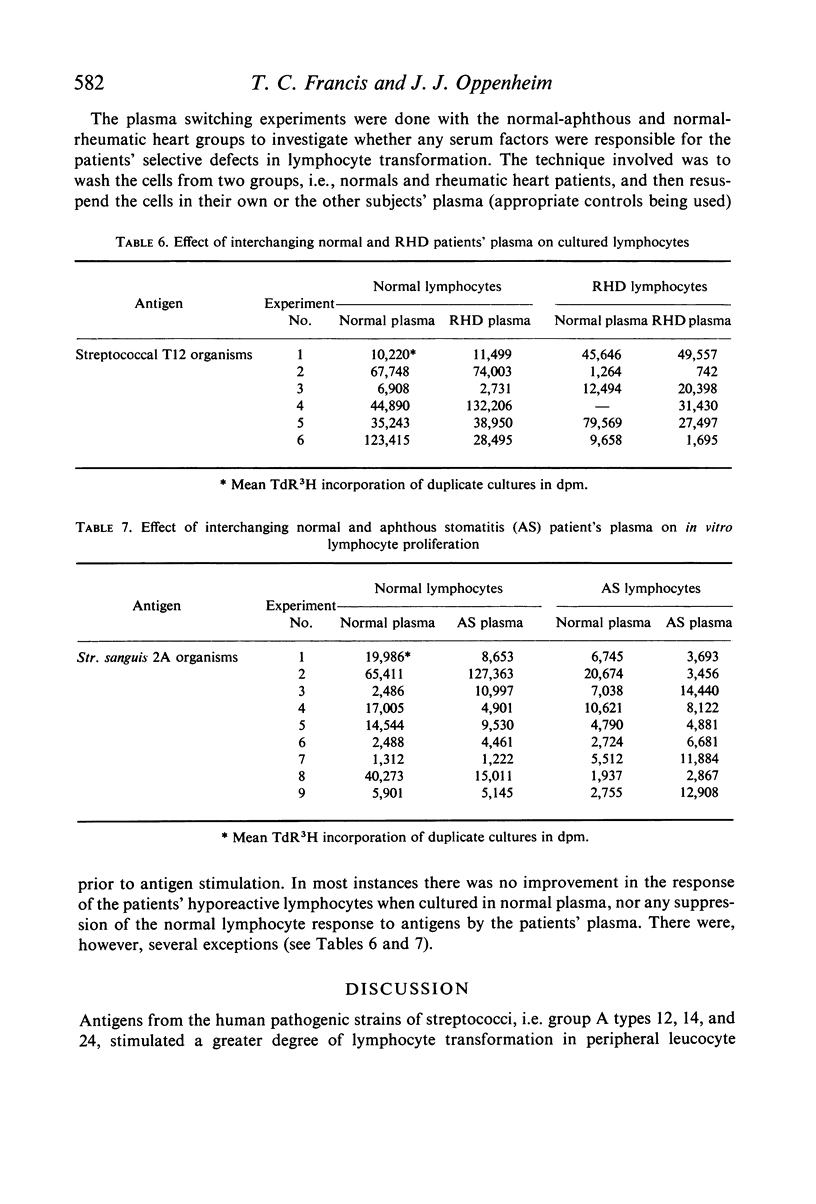
The effects of pathogenic and non-pathogenic streptococci, streptococcal cell wall products, and phytohaemagglutinin on human peripheral leucocyte cultures from four groups were studied. These groups were: (1) normals, (2) patients with aphthous stomatitis, (3) patients with Behçet's and (4) patients with rheumatic heart disease. The degree of lymphocyte stimulation by these materials was measured by uptake of [3H]thymidine into DNA in vitro. In normals, patients with aphthous stomatitis, and Behçet's disease, the human pathogenic group A streptococci produced significantly greater stimulation of DNA synthesis than did the less pathogenic non-haemolytic streptococci. Lymphocytes from patients with aphthous stomatitis showed significantly less stimulation of DNA synthesis than comparable normal controls when exposed to heat-killed Streptococcal 2A, organisms which have been implicated in the disease. Human pathogenic strains of group A streptococci which have been implicated in rheumatic heart disease stimulated significantly less in vitro proliferation of lymphocytes from patients with rheumatic heart disease than of those from a comparable group of normal controls. This hypo-responsiveness persisted when the patients' lymphocytes were cultured in normal human serum. The chronically ill Behçet's patients' lymphocytes did not differ significantly from normal. These observations indicate a deficiency of the cellular response of certain patients to antigens from organisms thought to be aetiologically related to their disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dudding B. A., Ayoub E. M. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968 Nov 1;128(5):1081–1098. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K., Dorfman A. Antigenicity of the M proteins of group A hemolytic streptococci. 3. Antibody responses and cutaneous hypersensitivity in humans. J Exp Med. 1966 Dec 1;124(6):1135–1151. doi: 10.1084/jem.124.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graykowski E. A., Barile M. F., Lee W. B., Stanley H. R., Jr Recurrent aphthous stomatitis. Clinical, therapeutic, histopathologic, and hypersensitivity aspects. JAMA. 1966 May 16;196(7):637–644. doi: 10.1001/jama.196.7.637. [DOI] [PubMed] [Google Scholar]
- HIRSCHHORN K., SCHREIBMAN R. R., VERBO S., GRUSKIN R. H. THE ACTION OF STREPTOLYSIN S ON PERIPHERAL LYMPHOCYTES OF NORMAL SUBJECTS AND PATIENTS WITH ACUTE RHEUMATIC FEVER. Proc Natl Acad Sci U S A. 1964 Nov;52:1151–1157. doi: 10.1073/pnas.52.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., PAGEL W. The tissue response to heat-killed streptococci in the skin of normal subjects, and in persons with rheumatic fever, rheumatoid arthritis, subacute bacterial endocarditis and erythema nodosum. Br J Exp Pathol. 1949 Aug;30(4):282-8, 2 pl. [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H. The nature of antistreptolysin S in the sera of man and of other species; antistreptolysin titres in normal and diseased states. Br J Exp Pathol. 1949 Aug;30(4):345–351. [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE H. S. The cellular transfer in humans of delayed cutaneous reactivity to hemolytic streptococci. J Immunol. 1952 Feb;68(2):159–178. [PubMed] [Google Scholar]
- LEHNER T. RECURRENT APHTHOUS ULCERATION AND AUTOIMMUNITY. Lancet. 1964 Nov 28;2(7370):1154–1155. doi: 10.1016/s0140-6736(64)92673-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehner T. Stimulation of lymphocyte transformation by tissue homogenates in recurrent oral ulceration. Immunology. 1967 Aug;13(2):159–166. [PMC free article] [PubMed] [Google Scholar]
- Loewi G., Temple A., Vischer T. L. The immunological significance in the guinea-pig of in vitro transformation of lymph node, spleen and peripheral blood lymphocytes. Immunology. 1968 Feb;14(2):257–264. [PMC free article] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- OPPENHEIM J. J., PERRY S. EFFECTS OF ENDOTOXINS ON CULTURED LEUKOCYTES. Proc Soc Exp Biol Med. 1965 Apr;118:1014–1019. doi: 10.3181/00379727-118-30033. [DOI] [PubMed] [Google Scholar]
- Oettgen H. F., Silber R., Miescher P. A., Hirschhorn K. Stimulation of human tonsillar lymphocytes in vitro. Clin Exp Immunol. 1966 Jan;1(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- STOLLERMAN G. H., BERNHEIMER A. W. Inhibition of streptolysin S by the serum of patients with rheumatic fever and acute streptococcal pharyngitis. J Clin Invest. 1950 Sep;29(9):1147–1155. doi: 10.1172/JCI102352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranta A., Cuppari G., Quagliata F. Dissociation of hemolytic and lymphocyte-transforming activities of streptolysin S preparations. J Exp Med. 1969 Apr 1;129(4):605–622. doi: 10.1084/jem.129.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON M. G., SCHWEITZER M. Pattern of hereditary susceptibility in rheumatic fever. Circulation. 1954 Nov;10(5):699–704. doi: 10.1161/01.cir.10.5.699. [DOI] [PubMed] [Google Scholar]


