Abstract
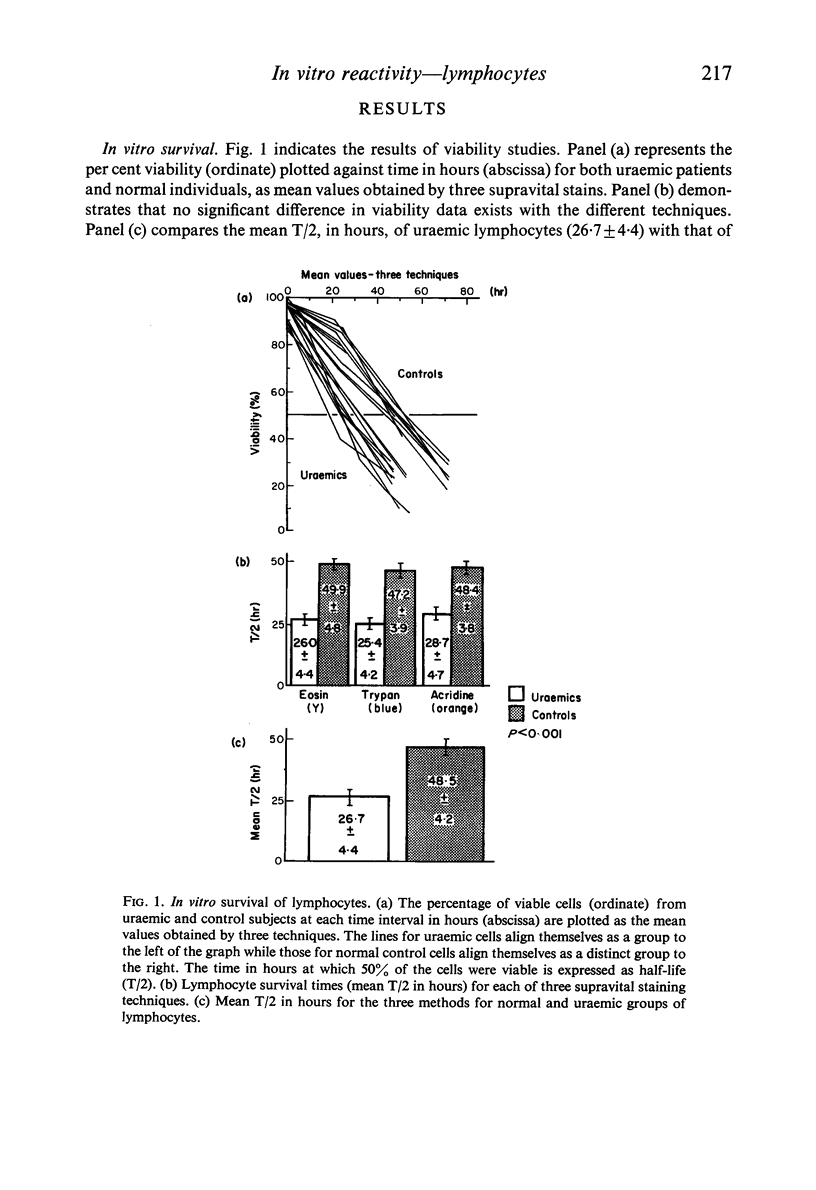
Altered cellular immunity complicating chronic uraemia includes lymphocytopenia, thymic atrophy, impaired allograft rejection and delayed hypersensitivity in skin tests, diminished appearances of lymphocytes on skin windows, and shortened in vitro survival of uraemic lymphocytes. Studies were undertaken to further characterize these lymphocyte defects.
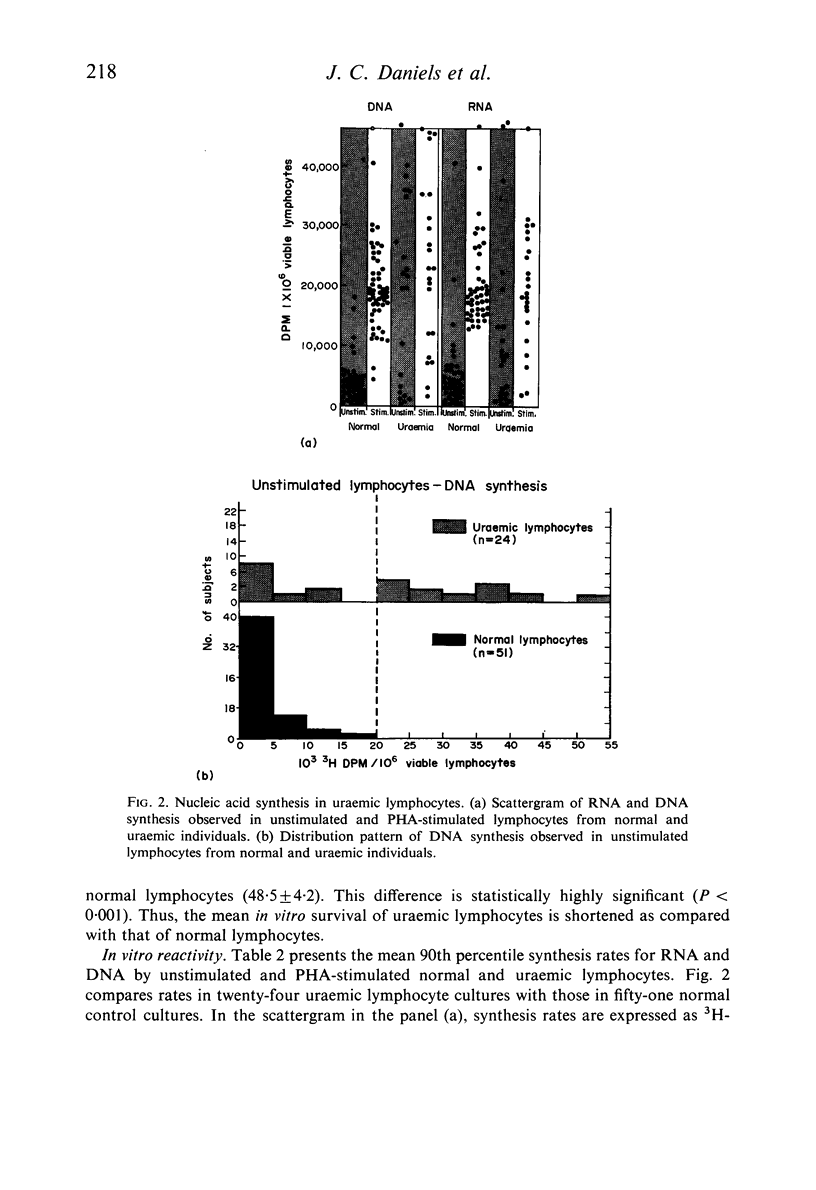
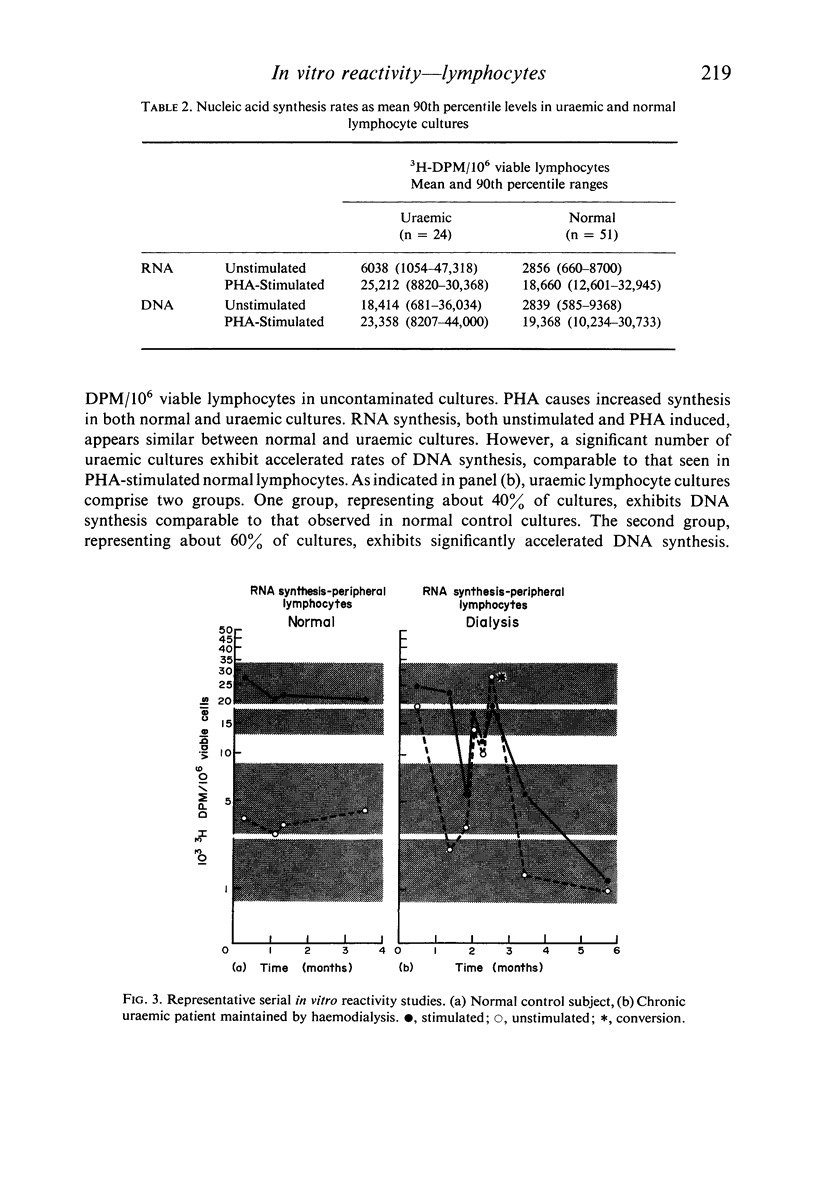
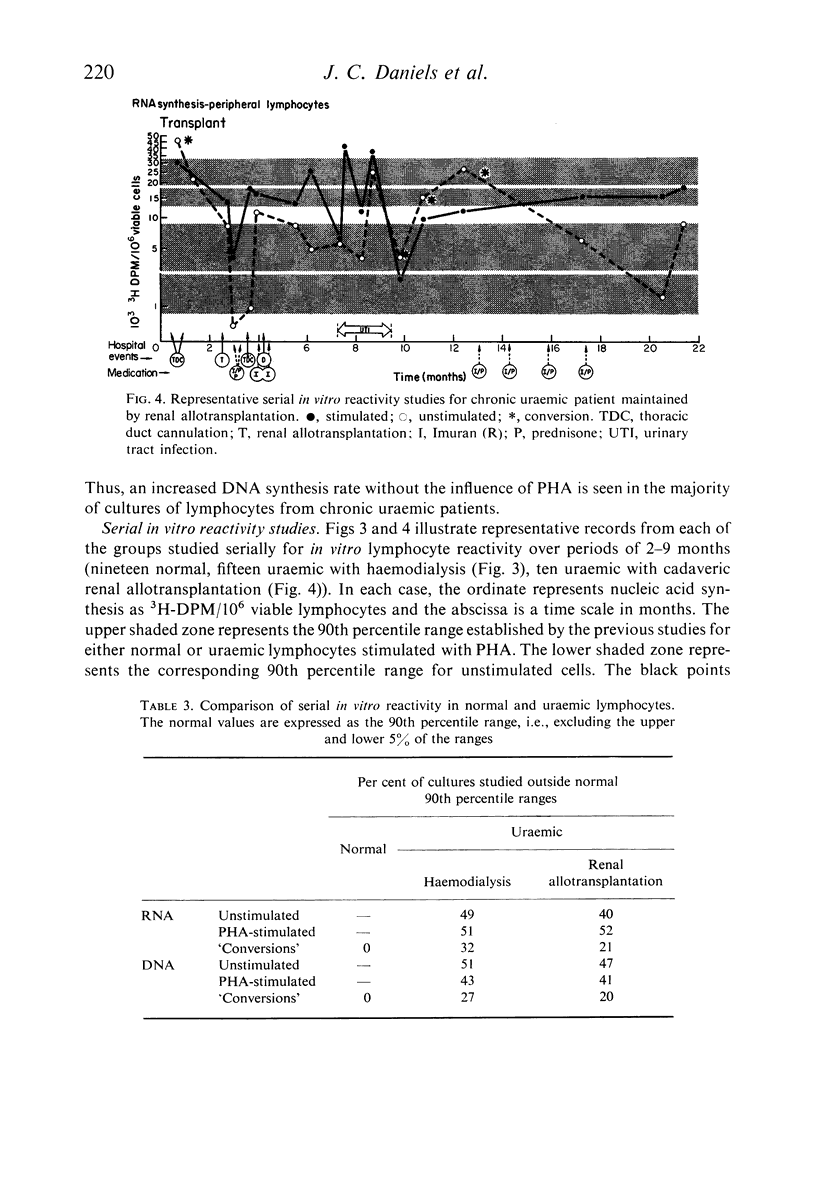
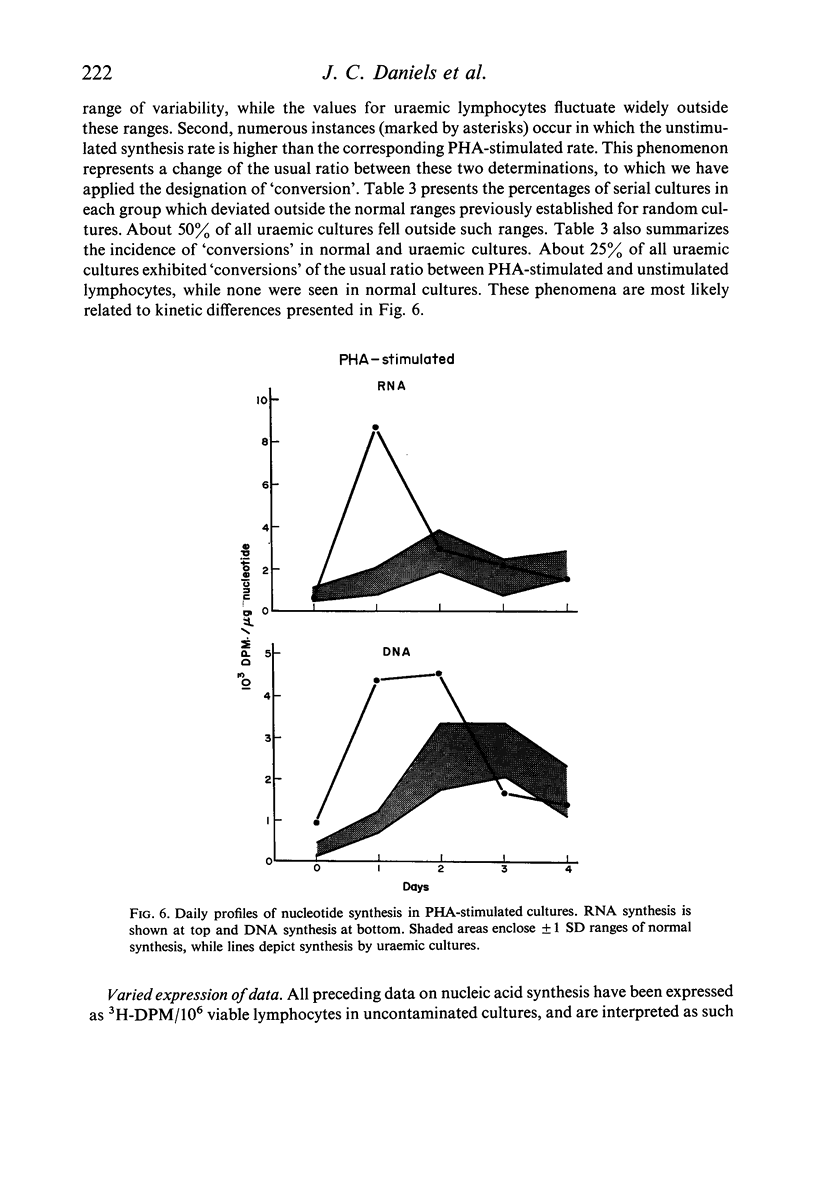
Lymphocytes separated from peripheral blood of twenty-four uraemic patients were compared with those from fifty-one normal persons in their rates of RNA and DNA synthesis in both PHA-stimulated and unstimulated cultures, as determined by incorporation of radiolabelled precursors. Synthesis, expressed as absolute incorporation/106 viable lymphocytes, was accelerated in the majority of both stimulated and unstimulated uraemic cultures. Serial studies over several months of twenty-five uraemic patients (fifteen maintained by haemodialysis, ten by renal allotransplantation), compared with nineteen normal controls, showed these differences to be consistent and persistent. While normal lymphocytes exhibited stability, uraemic cells fluctuated widely in their serial synthesis rates. In 25% of such cultures, unstimulated synthesis exceeded that induced by PHA. Increased synthesis without PHA, reflecting enhanced spontaneous blastogenesis, is compatible with decreased survival, numbers, and functional capacity of uraemic lymphocytes.
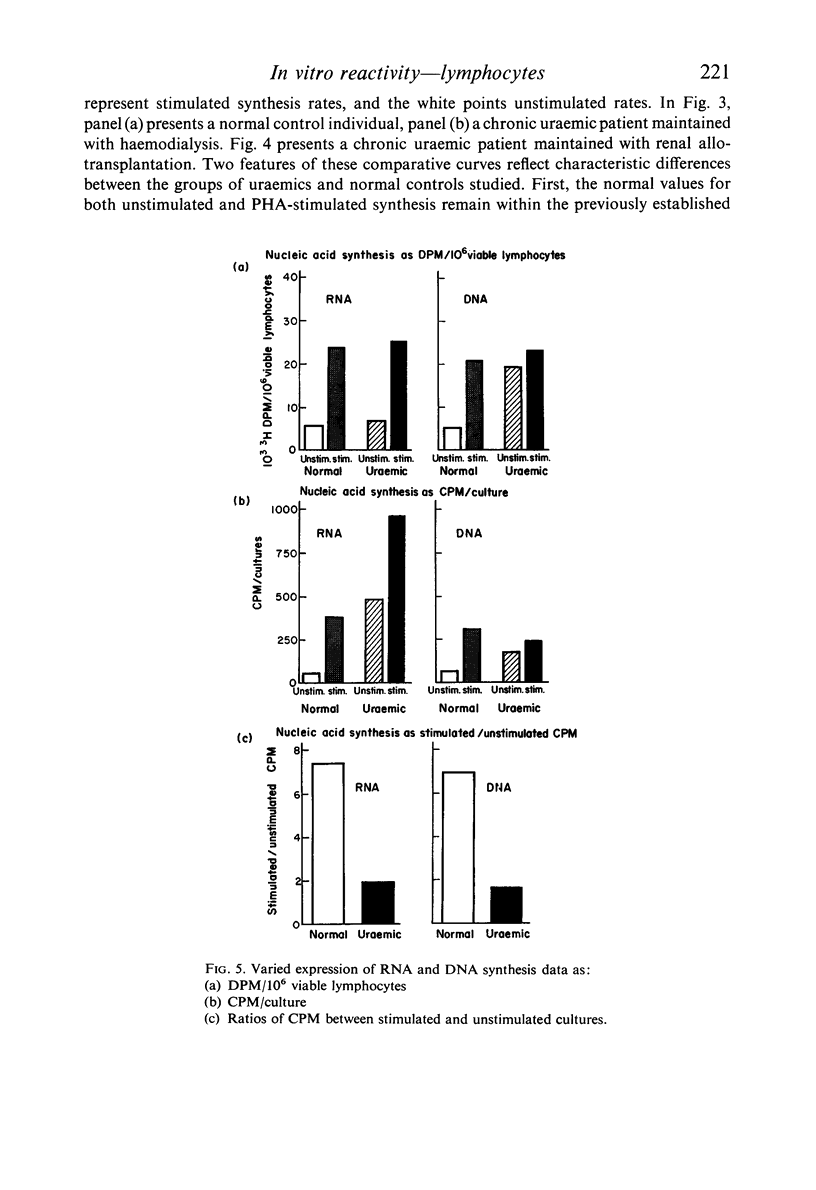
Reports by others of diminished PHA-responsiveness of uraemic lymphocytes are based upon whole-culture incorporation and/or expressed as ratios between stimulated and unstimulated cultures. Both shortened survival and accelerated spontaneous nucleic acid synthesis by uraemic lymphocytes cause such ratios to be misleadingly low. Such factors as cell numbers and viability at harvest, counting efficiency, and culture sterility are essential to avoid misinterpretations of such data based on incorporation of radiolabelled precursors to the nucleic acids.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH F. H., HIRSCHHORN K. THE IN VITRO IMMUNE RESPONSE OF PERIPHERAL BLOOD LYMPHOCYTES. Semin Hematol. 1965 Jan;2:68–89. [PubMed] [Google Scholar]
- BRIDGES J. M., NELSON S. D., MCGEOWN M. G. EVALUATION OF LYMPHOCYTE TRANSFER TEST IN NORMAL AND URAEMIC SUBJECTS. Lancet. 1964 Mar 14;1(7333):581–584. doi: 10.1016/s0140-6736(64)91331-5. [DOI] [PubMed] [Google Scholar]
- DAMMIN G. J., COUCH N. P., MURRAY J. E. Prolonged survival of skin homografts in uremic patients. Ann N Y Acad Sci. 1957 Mar 22;64(5):967–976. doi: 10.1111/j.1749-6632.1957.tb52488.x. [DOI] [PubMed] [Google Scholar]
- Dameshek W. Chronic lymphocytic leukemia--an accumulative disease of immunolgically incompetent lymphocytes. Blood. 1967 Apr;29(4 Suppl):566–584. [PubMed] [Google Scholar]
- Daniels J. C., Cobb E. K., Lynch J. B., Lewis S. R., Larson D. L., Ritzmann S. E. Altered nucleic acid synthesis in lymphocytes from patients with thermal burns. Surg Gynecol Obstet. 1970 May;130(5):783–788. [PubMed] [Google Scholar]
- Elves M. W., Israëls M. C., Collinge M. An assessment of the mixed leucocyte reaction in renal failure. Lancet. 1966 Mar 26;1(7439):682–685. doi: 10.1016/s0140-6736(66)91628-x. [DOI] [PubMed] [Google Scholar]
- HATHAWAY W. E., NEWBY L. A., GITHENS J. H. THE ACRIDINE ORANGE VIABILITY TEST APPLIED TO BONE MARROW CELLS. I. CORRELATION WITH TRYPAN BLUE AND EOSIN DYE EXCLUSION AND TISSUE CULTURE TRANSFORMATION. Blood. 1964 Apr;23:517–525. [PubMed] [Google Scholar]
- Huber H., Pastner D., Dittrich P., Braunsteiner H. In vitro reactivity of human lymphocytes in uraemia--a comparison with the impairment of delayed hypersensitivity. Clin Exp Immunol. 1969 Jul;5(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- KIRKPATRICK C. H., WILSON W. E., TALMAGE D. W. IMMUNOLOGIC STUDIES IN HUMAN ORGAN TRANSPLANTATION. I. OBSERVATION AND CHARACTERIZATION OF SUPPRESSED CUTANEOUS REACTIVITY IN UREMIA. J Exp Med. 1964 May 1;119:727–742. doi: 10.1084/jem.119.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P. A., Ritzmann S. E., Merian F. L., Lawrence M. C., Levin W. C., Gregory R. Cellular evolution in induced inflammation in uremic patients. Tex Rep Biol Med. 1966 Spring;24(1):107–111. [PubMed] [Google Scholar]
- Scheurlen P. G., Baake M., Frey N., Moers P., Sieberth G. Uber Immuninsuffizienz bei chronischen, urämischen Nierenerkrankungen. Dtsch Med Wochenschr. 1969 Jan 3;94(1):17–passim. doi: 10.1055/s-0028-1108893. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Thomson A. E. Death and division of lymphocytes. Neglected factors in assessment of P.H.A.-induced transformation. Lancet. 1968 Nov 23;2(7578):1120–1123. doi: 10.1016/s0140-6736(68)91583-3. [DOI] [PubMed] [Google Scholar]


